LOYOLA COLLEGE (AUTONOMOUS), CHENNAI – 600 034
advertisement
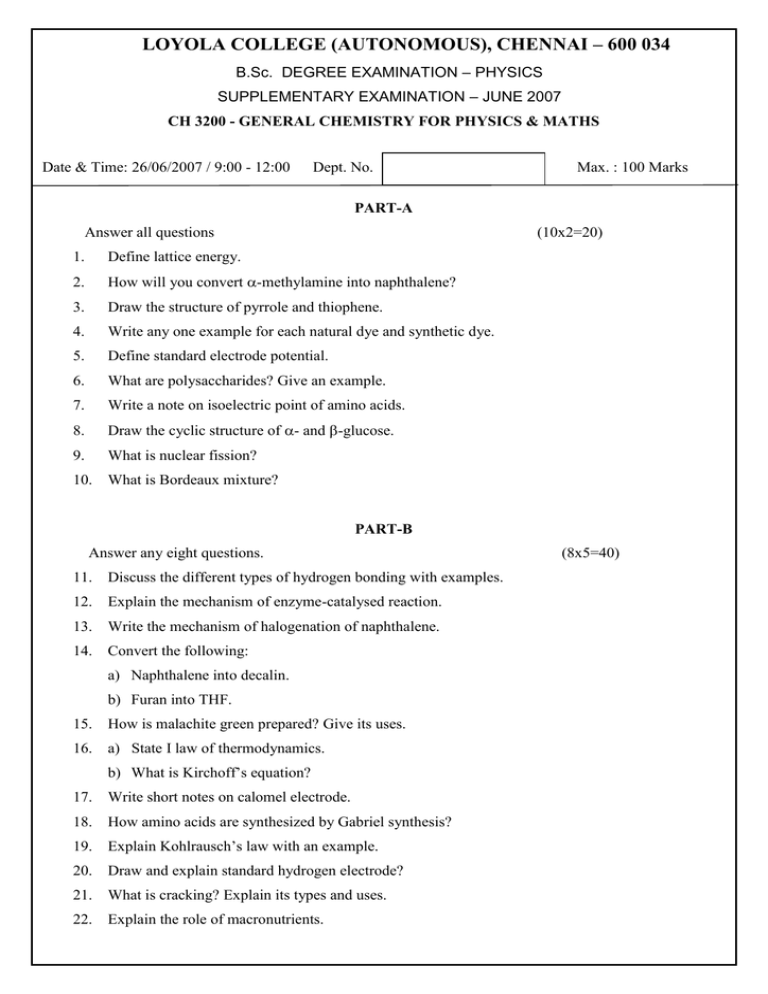
LOYOLA COLLEGE (AUTONOMOUS), CHENNAI – 600 034 B.Sc. DEGREE EXAMINATION – PHYSICS SUPPLEMENTARY EXAMINATION – JUNE 2007 CH 3200 - GENERAL CHEMISTRY FOR PHYSICS & MATHS Date & Time: 26/06/2007 / 9:00 - 12:00 Dept. No. Max. : 100 Marks PART-A Answer all questions (10x2=20) 1. Define lattice energy. 2. How will you convert -methylamine into naphthalene? 3. Draw the structure of pyrrole and thiophene. 4. Write any one example for each natural dye and synthetic dye. 5. Define standard electrode potential. 6. What are polysaccharides? Give an example. 7. Write a note on isoelectric point of amino acids. 8. Draw the cyclic structure of - and -glucose. 9. What is nuclear fission? 10. What is Bordeaux mixture? PART-B Answer any eight questions. 11. Discuss the different types of hydrogen bonding with examples. 12. Explain the mechanism of enzyme-catalysed reaction. 13. Write the mechanism of halogenation of naphthalene. 14. Convert the following: a) Naphthalene into decalin. b) Furan into THF. 15. How is malachite green prepared? Give its uses. 16. a) State I law of thermodynamics. b) What is Kirchoff’s equation? 17. Write short notes on calomel electrode. 18. How amino acids are synthesized by Gabriel synthesis? 19. Explain Kohlrausch’s law with an example. 20. Draw and explain standard hydrogen electrode? 21. What is cracking? Explain its types and uses. 22. Explain the role of macronutrients. (8x5=40) PART-C Answer any four questions. (4x10=40) 23. Explain the ion exchange method for the separation of lanthanides. 24. a) Write a note on carcinogenesity of higher polyaromatic hydrocarbons. (4) b) Explain the preparation, properties and uses of congo-red and mordant dyes.(6) 25. 26. a) Write the structure and uses of sulphadiazine. (4) b) Elucidate the structure of sucrose. (6) a) Discuss the different types of conductometric titrations. (6) b) Explain the secondary structure of proteins. (4) 27. Write notes on open and cyclic structure of fructose. 28. a) Write short notes on renewable and non-renewable energy resources. (6) b) How is super phosphate of lime prepared? Explain. (4) **** 2