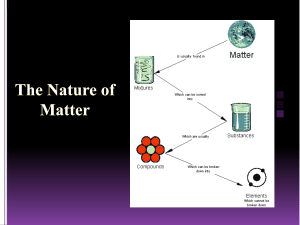
ICP Date: ____________ Name: ____________________ Atomic Structure Review Chapter 16, sect 1 and 2; Chapter 18, sect. 1 and 2 1. How many types of matter are in a mixture? 2. How many types of matter are in a pure substance? 3. Of a mixture or pure substance, which can be separated by physical means? 4. In what type of mixture is every sample going to be the same throughout? 5. In what type of mixture is every sample different from the other? 6. What is the type of pure substance that contains only one type of matter? 7. What is the type of pure substance that contains more than one type of matter? 8. What are the smallest particles of an element that still has its properties called? 9. What are the smallest particles of a compound that still has its properties called? 10. Label each of the following as being a(n): a. Compound c. Heterogeneous Mixture b. Element d. Homogeneous Mixture ____ copper ____ water ____ salt water ____ salt ____ Kool-Aid ____ sand, salt, gravel mixture 11. What two properties does all matter have? 12. What is the metric unit for measuring mass? 13. What is the metric unit for measuring space? 14. What are the charges of the three subatomic particles: a. Proton b. Electron c. Neutron 15. Which of the three subatomic particles are in the nucleus? ICP Date: ____________ Name: ____________________ 16. Which of the subatomic particles is much smaller than the others? 17. Are atoms mostly solid or mostly empty space? 18. What distinguishes one type of atom from another? 19. What does the atomic number of an element tell us? 20. What is the mass number of an atom? 21. Determine how many protons, neutrons and electrons are in the following atoms: a. Oxygen with a mass number of 16 b. Potassium with a mass number of 39 22. What are different atoms of the same element, but different masses called? 23. What subatomic particle can have different numbers in different atoms of the same element? 24. Can electrons be found anywhere in an atom, or only in a specific energy level? 25. Do electrons fill up energy levels from the lowest or highest levels first? 26. When an electron gains energy, will it jump to higher or lower energy levels? 27. When an electron loses energy, what is the energy released as? 28. Draw the electron energy levels and place the electrons in the appropriate level. a. Aluminum b. Potassium