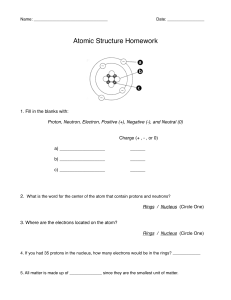
Name: _______________________________ Date ___________ Period _____ Periodic Table of the Elements Answer every question as completely as possible. ____ = 20 % 1. What are two properties of the noble gases group? (2 points) ________________________________________________________________ ________________________________________________________________ 2. If an atom has 118 protons in its nucleus, how many electrons does it have? (2 point) ________________________________________________________________ 3. What four pieces of information are given on every square of the periodic table? (4 points) ________________________________________________________________ ________________________________________________________________ ________________________________________________________________ ________________________________________________________________ 4. Why can’t you find elements 95 and up in nature? (3 points) ________________________________________________________________ ________________________________________________________________ ________________________________________________________________ 5. Why are alkali metals never found in nature? (3 points) ________________________________________________________________ ________________________________________________________________ ________________________________________________________________ ______ 6. An atom of _________ has a full set of electrons in its outer shell a. Alkali metal b. Halogen c. Noble Gas d. Alkaline-earth metal ______7. A ________ goes across the table from left to right: a. Family b. Group c. Period d. Tasty squirrel with ranch dressing _____ 8. How many electrons are in the third shell of an atom? a. 2 b. 8 c. 18 d. 32 _____ 9. Which of the following NOT found on the periodic table? a. The atomic number of each element b. The name of each element c. The date each element was discovered d. The atomic mass of each element _____ 10. Elements in the same up-down column belong to the same: a. Period b. Silicate c. Group d. Table _____ 11. The atomic mass shows: a. The number of protons b. The number of protons and electrons c. The number of neutrons d. The number of protons and neutrons