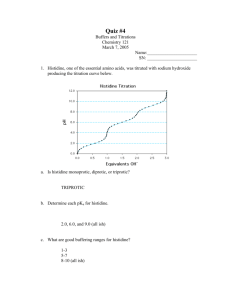
Amman Arab University Faculty of Pharmacy Practical Physical Pharmacy (71012107) 1st semester 2019/2020 Experiment 4 Phase Diagram- Mutual Solubility Curve for Phenol and Water OBJECTIVE To determine the solubility of 2 partially miscible liquids properties by using phenol in water INTRODUCTION A few liquids are miscible with each other in all proportions while others have miscibility under certain proportions only. A typical example for this is phenol and water. Under certain temperature and concentration of phenol and water, the phenol and water maybe in 1 phase condition or maybe being separated into 2 different phases. Generally, both liquids become more soluble with rising temperature until the critical solution temperature or consolute point is attained, and above that point, the liquids will turn into only 1 phase. At any temperature below the critical solution temperature, the composition for the 2 layers of liquids in equilibrium state is constant and does not depend on the relative amount of these 2 phases. The mutual solubility for a pair of partially miscible liquids in general is extremely influenced by the presence of a third component. APPARATUS AND MATERIALS Phenol, water, thermometer, boiling tube rack, boiling tubes, pipette PROCEDURES 1. Mixture of phenol and water in boiling tubes was prepared in the way that phenol was added in water in various percentages to form 8%, 11%, 20%, 50%, 60%, 63% and 80%. 2. The total amount of 2 liquids in the boiling tubes was fixed to be 30 ml and the boiling tubes were labeled accordingly from A to G. 3. Then, the boiling tube A was heated in hot water and the mixture was stirred. 4. The temperature at which the turbid liquid became clear was recorded. 5.The boiling tube A was then being cooled gradually and the temperature at which the liquids became turbid again forming 2 separated layers was recorded. Then, boiling tube A was heated again and the average temperature for heating and cooling was recorded. 6. Finally, steps 3-5 were repeated for boiling tubes B to G. 7. A graph of temperature at complete miscibility against phenol composition in the different mixtures was plotted. Percentage of phenol % Volume of phenol Volume of water 8.0 11.0 20.0 50.0 63.0 80.0 2.4 3.3 6.0 15.0 18.9 24.0 27.6 26.7 24.0 15.0 11.1 6.0 Average temperature at which the turbid liquid becomes clear (C) REPORT 1) Graph temperature versus percentage of phenol in water 2) Discussion 3) Conclusion Average temperature at which the liquid becomes turbid (C) Experiment 5 Chemical Kinetics Hydrolysis of methyl acetate Chemical kinetics deals with: The rate at which chemical reactions take place The mechanism by which reactions take place with particular or emphasis on decomposition and stabilization of drug products The influence of various factors such as temperature, pressure, concentration, light and catalysts etc, on reaction rate. Rate and Order of the reactions Rates of the reaction: The velocity by which a chemical reaction occurs A→B+C The rate of the reaction = the rate by which A decrease with time = - dA/dt Or the rate by which B (C) increase with time = dB/dt (dC/dt) 1- Minus (-) indicates the decrease in the concentration over the time 2- Rate unit: conc./time (e.g. mcg/ml.hr) Orders of the reaction: The way by which the concentration of the reactants influences the rate of a chemical reaction, according to that, the reactions are classified into: 1- Zero order reaction 2- First Order reaction 3- Second order reaction Zero Order reactions: The amount of the reactant is decreasing over a specific time interval is constant In this type of reactions, the rate is constant; it did not depend on the concentration of the reactant Rate = dA/dt = - K…………… (K is a constant) Example: Time (hr) 0 1 2 3 4 Amount (mg) 300 250 200 150 100 To calculate the concentration at any time: Rate = K dA/dt = - K…………………………………..by integration [A] = [Ao] – K * t……………………………………………………. (1) [A]: The concentration of the reactant at any time [Ao]: Initial concentration of the reactant K: Zero order rates constant t: Time Concentration time profile in zero order kinetics From equation (1): The slope of the curve = - K Y intercept (The concentration of the reactant when t = 0) = [Ao] Half-life: The time needed to the initial concentration to drop to its one half [A] = [Ao] – K * t 0.5 [Ao] = [Ao] – K * t1/2 0.5 [Ao] = K * t1/2 t1/2 = 0.5 [Ao] K In zero order reactions: The rate of the reaction didn’t depend on the concentration of the drug but the half life depend on the concentration of the reactions First Order reactions A constant fraction of the reactant is consumed per unit time. The rate of the reaction is proportional to the amount of the reactant. Rate α [A] Example Time (hr) 0 1 2 3 4 Amount (mg) 300 270 243 218.7 196.8 To calculate the concentration of the reactant at any time: Rate α [A] Rate = K * [A] dA/dt = - K * [A]…………………………………………By integration [A] = [Ao] * e-K * t Concentration time profile in first order reactions (Conc. versus time) Note in this graph: 1- The relationship between [A] and the time is an exponential relationship 2- The slope differ from one point to another, so we can’t calculate the slope from this curve To estimate the value of rate constant from concentration versus time data using graph paper we would prefer a straight line equation. This can be achieved by taking the natural logarithm of both sides: [A] = [Ao] * e-K * t In [A] = In [Ao] – K * t………………………………………………….. (2) Concentration time profile in first order reactions (In conc. versus time on a rectilinear graph) From equation (2): The slope = - K Y intercept = In [Ao] To convert from natural logarithm to common logarithm: In X = 2.303 * log X So; In [A] = In [Ao] – K * t Log [A] = log [Ao] –K * t…………………………… (3) 2.303 From equation (3): Slope = - K/2.303 Y intercept = log [Ao] Concentration time profile in first order reactions (Conc. versus time on a semi log graph) Half life: To calculate the half life for first order reactions: In [A] = In [Ao] – K * t In 0.5 [Ao] = In [Ao] – K * t1/2 K * t1/2 = In [Ao] – In 0.5 [Ao] K * t1/2 = In ([Ao]/ 0.5 [Ao]) K * t1/2 = In 2 t1/2 = In 2 / K = 0.693 / K In first order reactions: 1- The rate of the reaction depends on the concentration of the reactant but the half-life didn’t depend on the concentration 2- The unit of K is 1/time (Time -1) 3- Hydrolysis of methyl acetate Methyl acetate hydrolyzes to form acetic acid and methanol, according to the following reaction: The reaction is extremely slow in pure water, but is catalyzed by both hydronium and hydroxide ions. In this experiment the kinetics of the reaction catalyzed by HCl will be studied. Hydrolysis reaction of this type are reversible; the back titration is called esterification. For any equilibrium the overall rate is the difference in rates of the forward and reverse reaction. Procedure You will work in a specific temperature assigned by your instructor, carful pipetting and titrating techniques are essential if good data are to be obtained. 1. For the blank experiment, take 100 ml of 1 N HCl into a Stoppered flask and place it in the water bath at the selected temperature then Pipette 5ml into Erlenmeyer flasks containing about 75 ml of water, titrate with 0.2 N NaOH using phenolphthalein as indicator. 2. In volumetric flask, take 100 ml of 1 N HCl into Stoppered flask and place it in the water bath at the selected temperature. 3. Also place a flask of methyl acetate in the water bath at the same time, allow both to come to thermal equilibrium about 15 min.) 4. Pipette 5ml of the ester into the acid, record the time mixing. Sampling 1. As soon as possible withdraw 5ml aliquot of the reaction mixture Drain these samples into Erlenmeyer flasks containing about 75 ml of ice and water (to make dilution and slow down the hydrolysis). 2. Titrate the diluted aliquot as soon as possible with standard NaOH using phenolphthalein as indicator. 3. According to the time schedule given by your instructor repeat step 1 and 2. Note: The time of sampling is recorded as the time when approximately half of the sample has flowed from the sampling pipette. Delivering the sample into ice water slows down the reaction but the titration of the sample as soon after sampling as possible. NaOH pellets are CAUSTIC, use spatula for transferring (NO Hand); weigh out the necessary amount of pellets into glass container. Report sheet II Stability of drugs (hydrolysis of methyl acetate) Student name: Objectives: Results: Time (Min) Volumes (NaOH) Blank 0 5 10 15 20 ∆ V (Vsample – Vblank) No. Of moles of acetic acid --- --- Initial moles of methyl acetate Remaining moles of methyl acetate ---- Calculation and graph 1) In the above table write your own zero and sampling times then record the concentration at each time 2) Plot the results according to the instructor directions 3) Determine the reaction order 4) Determine the reaction rate constant Note: M.Wt for methyl acetate: 74.1g/mole Density for methyl acetate: 0.932g/ml Conclusion: --------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------- Plagiarism Warning! Some of these experiments are carried out in groups of usually a pair of students. Therefore, expectedly, each member of a group followed an identical procedure in the laboratory and has the same set of raw data. Members of a group are allowed to discuss the analysis of data with one another. However, preparation of the report including data analysis, interpretation and discussion must be prepared by the individual student submitting the report. The Department does not tolerate plagiarized report! Student name: Experiment 6 The Influence of Temperature on Reaction Rate Many factors (concentration, temperature, solvents, catalysts and light) may affect the reaction rate. It is well known fact that raising the temperature increases the reaction rate. The effect of temperature on the rate of decomposition may be described by Arrhenius equation: K= A e –Ea/RT Or log k = Log A - Ea 2.3030 RT Where; A: Frequency Factor or Arrhenius Factor (It is specific for each reaction). Ea: Activation energy (Energy when the collision of two reactants molecules is to lead to reaction). R: is the gas constant (1.987 calories/deg. Mole = 8.314 J/mole.K). T: is the absolute temperature (Kelvin K). Two point form of Arrhenius Equation The activation energy can also be found by substituting two rate constants (K1, K2) and the two corresponding reaction temperatures (T1, T2) into Arrhenius equation Log K1 = Ea * K2 2.303R T1 - T2 T1*T2 Student name: Hydrolysis of aspirin Aspirin [C9H8O4 (MWt. = 180.16 g/mole)] have the following properties: a. It is used as analgesic, antipyretic and anti-inflammatory. b. It is an ester and acid. c. Should be stored in tight containers. d. Hydrolysis of aspirin is first- order reaction. e. A deep violet color is obtained upon addition of FeCl3. COOH O O PH = 10 CH3 O + H2O + O Aspirin O O CH3 O Salicylate ion Acetate ion Experimental part: 1. Transfer 1.00 ml of alcohol solution of aspirin (0.15 g/100ml) into 4 test tubes. 2. Add 9.00 ml of distilled water to each test tube and record time. 3. Transfer the tubes into water bath adjust at 400C, 60oC, 70oC and 90 oC. 4. Every 10 min, take 1ml from the test tube in the water bath and put it in a new tube, cool in ice bath for 2-3min, and then add 1.00 ml of FeCl3 solution, mix will and measure the absorbance λmax=523.5nm. 5. Calculate the amount of salicylic acid from Beers low. Student name: Report sheet 6 Objectives: --------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------- Results: At 40°C: Time Absorbance Concentration (M) (Hydrolyzed) Concentration of Remained Aspirin (Initial –hydrolyzed) Log [C] Absorbance Concentration (M) (Hydrolyzed) Concentration of Remained Aspirin (Initial –hydrolyzed) Log [C] Absorbance Concentration (M) (Hydrolyzed) Concentration of Remained Aspirin (Initial –hydrolyzed) Log [C] 10 20 30 At 60°C: Time 10 20 30 At 70°C: Time 10 20 30 Student name: At 90°C: Time Concentration (M) (Hydrolyzed) Absorbance Concentration of Remained Aspirin (Initial –hydrolyzed) Log [C] 10 20 30 Temperature (°C) 40 60 70 90 K 1/T Log K Log K + 2 (to remove minus sign) Notes: A. Absorptivity of S.A. FeCl3 Complex = 426L.mol-1.cm-1. B. M.Wt. of A.S.A= 180.00 g/mole C. Concentration of A.S.A stock solution= 0.15 g/100ml or 8.31x10-3 M. Calculation and graphs Plot the X-axis (time (minutes)) against Y-axis ([C]) for A.S.A and attached it with report. Find the order of the reaction, the rate constant K, slope and t ½. Plot the data according to Arrhenius equation and compute activation energy and frequency factor using the Arrhenius equation. Conclusion: --------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------------- Rate of hydrolysis of aspirin Student worksheet Health and safety note Wear eye protection. Hydrolysis of aspirin Aspirin (2-ethanoyloxybenzoic acid or acetylsalicylic acid) hydrolyses to produce 2-hydroxybenzoic acid and ethanoic acid. Here is the equation for the reaction: O H3C OH O O OH HO H + O OH O + H H3C C O The rate at which this reaction happens is important for two reasons. When administered, aspirin hydrolyses in the body. Also, if aspirin tablets are stored in a humid environment, such as a bathroom, they hydrolyse. The amount of 2-hydroxybenzoic acid produced can be determined form the intensity of the violetblue complex it forms with iron(III) ions. Equipment and materials Colorimeter and suitable filter (green/yellow); a solution of the complex displays maximum absorption at 530 nm. Thermometer Colorimeter cuvette/tube, able to hold 6 cm3 5 cm3 and 1 cm3 graduated pipettes or syringes 100 cm3 conical flask Powdered aspirin − Harmful 0.025 mol dm−3 iron(III) nitrate solution pH 7.5 buffer solution Magnetic stirrer-hotplate and flea Method 1. Measure out 100 cm3 of the pH 7.5 buffer solution into a conical flask. Put this on a magnetic stirrer/electric hotplate and bring the temperature up to 70 °C. 2. Add 0.10 g of powdered aspirin to the buffer solution and stir the mixture gently until the aspirin is completely dissolved. No measurements should be made until all the solid has dissolved. 3. Pipette 5 cm3 of iron(III) nitrate reagent directly into a colorimeter tube. Pipette 1 cm3 from the reaction mixture and empty it into the colorimeter tube. Mix well and measure the absorbance. The intensity of the colour and, therefore, the absorbance value depends on the concentration of the hydrolysis product 2-hydroxybenzoic acid. Note: The first reading represents "time 0" although it is likely that some decomposition will have already occurred. 1 4. Take 1 cm3 samples every 15 minutes for at least 2 hours and treat them as in step 4. 5. Use the calibration graph produced in Determination of 2-hydroxybenzoic acid to calculate the concentration of 2-hydroxybenzoic acid in solution at each time interval. Processing results The rate equation for the hydrolysis of aspirin is complex, but at a given pH in dilute aqueous solution the equation reduces to the first-order equation: where, [A] is the concentration of aspirin k is the rate constant (its value depends on temperature and pH). 1. From the concentration of aspirin, calculate the concentration of aspirin remaining in solution. 2. Plot a graph of aspirin concentration, [A], against time. 3. Measure the tangent at various point on the [A] vs time graph. These give the rate of reaction at various aspirin concentrations. 4. Plot a graph of rate of reaction against aspirin concentration. The slope of this graph gives the value of the rate constant, k, under the reaction conditions used. 5. The half-life of a reaction is the time it takes for the concentration of a reactant to halve or the concentration of a product to double. For a first order reaction the half-life is a constant value. No matter what the starting concentration of a reactant it takes the same time for this concentration to halve. From your results, work out the half-life for the hydrolysis of aspirin. Possible extension ideas The effects of pH and temperature on the rate of hydrolysis could be investigated. 2 Experiment 7 Adsorption isotherm Adsorption is a process that occurs when a gas or liquid solute accumulates on the surface of a solid or a liquid (adsorbent), forming a molecular or atomic film (the adsorbate). It is different from absorption, in which a substance diffuses into a liquid or solid to form a solution. The term sorption encompasses both processes, while desorption is the reverse process. Adsorption is operative in most natural physical, biological, and chemical systems, and is widely used in industrial applications such as activated charcoal, synthetic resins and water purification. Similar to surface tension, adsorption is a consequence of surface energy. In a bulk material, all the bonding requirements (be they ionic, covalent or metallic) of the constituent atoms of the material are filled. But atoms on the (clean) surface experience a bond deficiency, because they are not wholly surrounded by other atoms. Thus it is energetically favorable for them to bond with whatever happens to be available. The exact nature of the bonding depends on the details of the species involved, but the adsorbed material is generally classified as exhibiting physisorption or chemisorption. 1-Physisorption Physisorption or physical adsorption is a type of adsorption in which the adsorbate adheres to the surface only through Van der Waals (weak intermolecular) interactions so it’s reversible desorption by increasing temp., and reducing pressure , which are also responsible for the non-ideal behavior of real gases. 2-Chemisorption Chemisorption is a type of adsorption whereby a molecule adheres to a surface through the formation of a chemical bond, as opposed to the Van der Waals forces which cause physisorption. The relationship between the amounts of gases physically adsorbed on a solid and the equilibrium pressure or concentration at constant temp., yields an adsorption isotherm. One can find in literature several models describing process of adsorption, namely Freundlich isotherm, Langmuir isotherm, BET isotherm, etc. Langmuir isotherm In 1916, Irving Langmuir published an isotherm for gases adsorbed on solids, which retained his name. It is an empirical isotherm derived from a proposed kinetic mechanism. It is based on four hypotheses: 1. The surface of the adsorbent is uniform, that is, all the adsorption sites are equal. 2. Adsorbed molecules do not interact. 3. All adsorption occurs through the same mechanism. 4. At the maximum adsorption, only a monolayer is formed: molecules of adsorbate do not deposit on other, already adsorbed, molecules of adsorbate, only on the free surface of the adsorbent. For liquids (adsorbate) adsorbed on solids (adsorbent), the Langmuir isotherm (Fig. 1). (Fig. 1) the Langmuir isotherm Langmuir equation X/m = (abC) / (1+bC) (1) This can be written as: C / (X/m) =1/ab + (1/a) C (2) When equation (2) give linear plot of C / (X/m) versus C, monolayer adsorption is assumed. In the above equation: X = amount of solute adsorbed per weight, m, adsorbent C = concentration of solution at equilibrium a,b = constant related to the system Freundlich isotherm (multilayer) The Freundlich equation or Freundlich adsorption isotherm is an adsorption isotherm, which a curve is relating the concentration of a solute on the surface of an adsorbent, to the concentration of the solute in the liquid with which it is in contact. Freundlich equation (multilayer) X/m = k *p1/n log X/m = log k + 1/n log P (3) (4) When equation (4) give linear plot of Log X /m versus log C, multilayer adsorption is assumed. Where X is the weight of the gas adsorbed by m gm of the adsorbent at a pressure p, X/m represents the amount of gas adsorbed by the adsorbents per gm (unit mass), K and n are constant at a particular temperature and for a particular adsorbent and adsorbate (gas), n is always greater than one, indicating that the amount of the gas adsorbed does not increase as rapidly as the pressure. Activated charcoal Activated carbon, also called activated charcoal or activated coal, is a general term that includes carbon material mostly derived from charcoal. For all three variations of the name, "activated" is sometimes substituted by "active." By any name, it is a material with an exceptionally high surface area. Just one gram of activated carbon has a surface area of approximately 500 m² (for comparison, a tennis court is about 260 m²). The three main physical carbon types are granular, powder and extruded (pellet). All three types of activated carbon can have properties tailored to the application. Activated carbon is frequently used in everyday life, in: industry, food production, medicine, pharmacy, military, etc. In pharmacy, activated charcoal is considered to be the most effective single agent available as an emergency decontaminant in the gastrointestinal tract. It is used after a person swallows or absorbs almost any toxic drug or chemical. Procedure 1. To 4 clean and dry Stoppered flasks transfer 1gm of charcoal. 2. Transfer accurately 100 ml of 0.1, 0.05, 0.003 and 0.025 M acetic acid solutions to the charcoal flasks 3. Shake the flasks well for 15 min. allow standing for 10 min. 4. Filter through filter paper, discard the first 10 ml. 5. Take 10 ml from pure filtrate and titrate with 0.05 M NaOH using phenolphthalein as indicator Report sheet 7 Adsorption isotherm Student name: Objectives: Results: A. complete the following table Concentration of solution (M) Volume of 0.05 M NaOH (ml) Moles of free acetic acid in 10ml Moles of free acetic acid in 100ml Initial moles of acetic acid Adsorbed Weight of moles of charcoal acetic acid (m) (X) 0.1 0.05 0.033 0.025 Ceq (M) x/m (moles of free acetic acid in 100ml / volume) 1g Ceq / (x/m) Log Ceq Log x/m Calculation and graph 1. From the titration results, calculate the equilibrium concentration of acetic acid from each sample, Ceq. 2. Calculate the number of mole present before and after adsorption 3. Calculate the number of adsorbed moles per one gm of charcoal X/m 4. Using normal graph paper plot Ceq/(x/m) Vs Ceq 5. Using semilog paper plot log(X/m) Vs log (Ceq) 6. It is Freundlich or Langmuir relationship? 7. From the graph with straight line relationship calculate the slope and the intercept of the graph depending on the type of the obtained isotherm. Experiment 8 Preparation of Buffers The pH (hydrogen ion potential) of a solution is defined as pH = -log10 (H+), where (H+) is the hydrogen ion concentration, a neutral solution has pH 7.0. For a weak acid, which dissociates as follows: HA H+ + A- An interesting and extremely useful relationship between pH and pKa can be obtained simply by taking logarithms (to the base 10) of the above: log10Ka = log10[H+] + log10[A- ] - log10[HA] Therefore -log10[H+] = -log10Ka + log10[A-] - log10[HA] Giving the Henderson-Hasselbalch equation: The most convenient form of this Henderson-Hasselbalch equation, is By using pKa values, we are able to express the strength of an acid (i.e. its tendency to dissociate) with reference to the pH scale. If Ka, the dissociation constant, is large, then pKa will have a low numerical value. A strong acid is one which is largely, perhaps completely, dissociated, and which therefore has a high Ka value. A weak acid is one that is only slightly dissociated in solution, and has a low Ka value. pKa values are used both for acids and for the conjugate acids of bases. Thus NH 3 has pKa (for the dissociation NH4+ H+ + NH3) about 9.3. There is no real need for Kb (and pKb) values, given by Kb = [BH+][HO-]/[B][H2O]. Should such values ever be required, for dilute aqueous solutions Kb = 10-14/Ka and pKb = 14.0 - pKa Where Kb and pKb refer to the conjugate acid (BH+ H+ + B). Perhaps it is useful to look at this in another way: if we consider the situation where the acid is one half dissociated, in other words where [A-] is equal to [HA], then, substituting in the Henderson-Hasselbalch Equation pH = pKaa + log10(1) Therefore pH = pKa + 0 Therefore pH = pKa This means that an acid is half dissociated when the pH of the solution is numerically equal to the pKa of the acid. Therefore acids with the lowest pKa values are able to dissociate in solutions of low pH, i.e. even where the hydrogen ion concentration is high. Acids with higher pKa values dissociate only in solutions of high (more alkaline) pH. Acid Ka pKa Trichloroacetic 2 x10-1 =10-0.7 0.7 Dichloroacetic 5 x10-2 =10-1.3 1.3 Monochloroacetic 1.6 x10-3 =10-2.8 2.8 Formic 2.1 x10-4 =10-3.7 3.7 Benzoic 7.8 x10-5 =10-4.1 4.1 Acetic 1.9 x10-5 =10-4.7 4.7 H2CO3 2.9 x10-7 =10-6.5 6.5 H2S 5.8 x10-8 =10-7.2 7.2 HCN 1.3 x10-9 =10-8.9 8.9 The table above gives some examples of Ka and pKa values for a number of acids, which are listed in order of decreasing strength. A buffer solution is an aqueous solution consisting of a mixture of a weak acid and its conjugate base or a weak base and its conjugate acid. It has the property that the pH of the solution changes very little when a small amount of strong acid or base is added to it. Buffer solutions are used as a means of keeping pH at a nearly constant value in a wide variety of chemical applications. Many life forms thrive only in a relatively small pH range; an example of a buffer solution is blood. Buffer is a mixture of dissolved substances which tends to keep the pH of a solution constant when modest additions of acid or base are made to that solution. No buffer can keep the pH exactly constant in such situations. The Henderson-Hasselbalch equation is very useful for calculations involving buffers, and is fairly accurate under reasonable conditions. Practical buffers consist of mixtures of weak acids and their salts. They will buffer solutions over a range of pH about 1 unit either side of the pKa value. Polybasic acids or mixtures of acids will have several pKa values, and (when mixed with their salts) can therefore buffer over several ranges of pH. Provided the individual pKa values differ by less than 2 units, the mixture will show more or less continuous buffering over a wide range of pH. For example, citric acid (pKa values 3.1, 4.8, and 6.4) mixed with one of its salts such as trisodium citrate will buffer continuously from pH 2 to 7.5, but not at higher pH values. The Henderson-Hasselbalch equation is used for the calculation of the pH or composition of a buffer solution. With mixtures consisting of weak acids (only slightly dissociated) and their salts (nearly totally ionised) the convenient approximations [HA] = total acid concentration, and [A-] = salt concentration, can often be made. This is adequate for most buffer design purposes; the exact pH required being obtained by adjusting the composition by adding a little strong acid or base. Hence an acetic acid/acetate buffer solution containing 0.1 M acetic acid and 0.05 M sodium acetate would have a pH of 4.4. pH = pKa + log10[conjugate base] = 4.7 + log10(0.05) = 4.7 + (-0.3) = 4.4 [conjugate acid] (0.1) Buffers function as follows: when a strong acid is added, the H+ from that acid combine with a portion of the anion to form undissociated acid, thereby removing most of the added H+ from the solution H+ + A- HA When strong base is added part of the undissociated acid reacts to form anions Base + HA A- + Base-H+ Useful buffer mixtures Components HCl, Sodium citrate Citric acid, Sodium citrate Acetic acid, Sodium acetate K2HPO4, KH2PO4 Na2HPO4, NaH2PO4 Borax, Sodium hydroxide pH range 1-5 2.5 - 5.6 3.7 - 5.6 5.8 - 8 6 - 7.5 9.2 - 11 Procedure Prepare 100 ml buffer solutions of 0.3 M acetic acid and sodium acetate of the following pHs 4, 4.5, 5.5 and 5.7 values using 1M acetic acid and 2 N NaOH stock solutions, knowing that the pKa of acetic acid is 4.75. Example Prepare 100 ml of pH = 6 from acetic acid and sodium acetate stock solution (1M each). Total molarity = 0.3M. pKa of acetic acid = 4.75