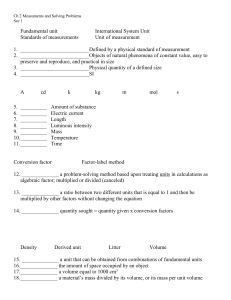
May 4 Gas Laws Notes Gases are very energetic and it’s molecules bounce around like crazy, called elastic collision Exerts pressure leads to what is called atmospheric pressure which can be measured with a barometer Pascals or (kPa) or the main measurement used then there is atmospheres or (atm) 760mmHg= 101.3 pascals= 1 atmosphere When changing pressure add or remove moles of gas change the volume and change the temperature as a whole Kelvin and celsius=+273 Celsius and kelvin=-273 p1v1=p2v2 P1 p2 T1 t2 V1 v2 T1 t2

![Temperature Notes [9/22/2015]](http://s3.studylib.net/store/data/006907012_1-3fc2d93efdacd086a05519765259a482-300x300.png)