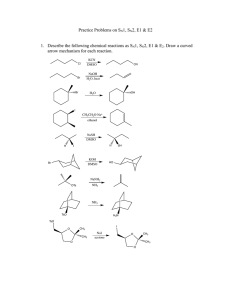
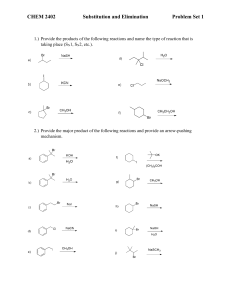
PRESENTED BYSHWETA PARIK M.Sc (I sem) 1.Aliphatic Nucleophilic Substitution and types 2.Mechanisms- introduction 3.The SN2 Mechanism 4.Evidences for the SN2 mechanism: 5.The SN1 Mechanism 6.Evidences for the SN1 Mechanism 7.THE SET Mechanism (single-electron transfer). 8.The SNi Mechanism 9.Mixed SN1 AND SN2 Mechanism 10.Nucleophilic Substitution at an Allylic Carbon 11.Nucleophilic Substitution at an Aliphatic Trigonal 12.Carbon Nucleophilic Substitution at a Vinylic Carbon ALIPHATIC NUCLEOPHILIC SUBSTITUTION SUBSTITUTION• replacement of an atom or group of atom by any other atom or group of atom. NUCLEOPHILE At least one unshared pair of electron Lewis base Attracted towards positively charged species. May be neutral or negatively charged. Example: Cl¯, Br¯, I¯, CN¯, OH¯,NH₃, RNH₂, H₂O, ROH, etc. General reactionR- X + Y: substrate nucleophile R-Y + X: substituted product Leaving group Nucleophile Y: may be neutral or negatively charged. Substrate may be neutral or positively charged. SN2 MECHANISM SN2 stands for substitution nucleophilic bimolecular. BACK SIDE ATTACK SINGLE STEP. Direct displacement. NO INTERMEDIATE FORMATION INVERSION OF CONFIGURATION The energy necessary to break the C-L bond is supplied by simultaneous formation of the C-Nu bond. GENERALLY YIELD SINGLE PRODUCT Evidences for the SN2 mechanism: 1.Kinetics: • rate expression, Rate= k[substrate][nucleophile] • If a large excess of nucleophile is present (i.e. solvolysis) – Rate= k [substrate] such kinetics are called pseudo-first order. 2. Stereochemistry: 3. No substitution at bridgehead carbon • Not be able to react by this mechanism, since the nucleophile cannot approach from the rear • REQUIREMENT FOR SN2: • SN2 mechanism requires the backside attack by the nucleophile, inversion of configuration and co planarity of three no-reacting groups in the transition state . • REASON: prevented at the bridge head carbons due to rigid cage like structures SN1 MECHANISM SN1 mechanism (substitution nucleophilic unimolecular) TWO STEP PROCESS The first step is a slow ionization of the substrate and is the rate-determining step The second is a rapid reaction between the intermediate carbonation and the nucleophile The ionization of a leaving group to form the carbocation is always assisted by the solvent, since the energy necessary to break the bond is largely recovered by solvation of CATION and of LEAVING GROUP. EVIDENCES FOR THE SN1 MECHANISM: 1. kinetics • first-order reaction following the rate law: RATE= k [RX] • solvent is necessary to assist in the process of ionization, it does not enter the rate expression, because it is present in large excess. 2. STEREOCHEMISTRY The stereo chemical evidence for the SN1 mechanism is less clearcut than it is for the SN2 mechanism. First-order substitutions do give complete racemisation, many others do not. Typically there is 5–20% inversion. • Some of the products are not formed from free carbocations but rather from ion pairs. • Role of ion pair: • Here is an intimate, contact, or tight ion pair; a loose or solvent-separated ion pair and the dissociated ions (each surrounded by molecules of solvent) are formed. The reaction products can result from attack by the nucleophile at any stage. Nucleophilic attack by a solvent molecule on tight ion pair thus leads to inversion. Attack on solvent separated ion pair leads to racemisation. 3. Formation of rearranged products: THE SET MECHANISM (singleelectron transfer). 4. NO substitution at bridgehead carbons • Not be able to react by this mechanism, since the nucleophile • REQUIREMENT: SN1 proceed through carbocations which must be planar. • REASON: Because of rigid cage like structure, bridgehead carbon atom cannot assume planarity, hence heterolysis leading to formation of carbocation is prevented. • In some nucleophilic reaction, sn1 is highly probable. • Esr detection of intermediate- free radicals are involved. • Carbocation- good electron acceptor • Nucleophile- good electron donor. • Mechanism- SET mechanism. For example, the reaction between the triphenylmethyl cation and tbutoxide ion:• Ph₃C⁺ + t-BuO¯ Ph₃C° + t-BuO° t-BuOCPh₃ Mixed SN1 AND SN2 MECHANISM:• Some reactions of a given substrate under a given set of conditions display all the characteristics of – • RX • SN1 IN BETWEENTWO THEORIES intermediate behaviour • • mechanism that is neither ‘‘pure’’ SN1 nor ‘‘pure’’ SN2, but some ‘‘in-between’’ type. all SN1 and SN2 reactions can be accommodated by one basic mechanism (the ion-pair mechanism). K2 2: simultaneous operation: • : INTERMEDIATE BEHAVIOUR- GIVEN BY SNEEN – K1 If k1>k2 then mechanism is SN2, if • k2> k1 then mechanism is SN1 • Borderline behaviour is found where the rates of formation and destruction of the ion pair are of the same order of magnitude (k1=k2). SN2 simultaneous operation The substrate first ionizes to an intermediate ion pair that is then converted to productsR⁺X¯ PRODUCT • no intermediate mechanism at all, and borderline behaviour is caused by simultaneous operation. in the same flask, of both the SN1 and SN2 mechanisms; that is, some molecules react by the SN1, while others react by the SN2 mechanism. • Among the experiments that have been cited for the viewpoint that borderline behaviour results from simultaneous SN1 and SN2 mechanisms is the behaviour of 4-methoxybenzyl chloride in 70% aqueous acetone. 4-methoxybenzyl chloride in 70% aqueous acetone. conversion to 4-methoxybenzyl alcohol occurs by an SN1 mechanism. alcohol is still a product, but now 4-methoxybenzyl azide is another product. increases the rate of ionization but decreases the rate of hydrolysis, (SN2 MECHANISMFOR AZIDE FORMATION) The Sni Mechanism (substitution nucleophilic internal) In a few reactions, nucleophilic substitution proceeds with retention of configuration, even where there is no possibility of a neighboring-group effect. Follows second order kinetics RARE The reaction between alchols and thionyl chloride has been studied exclusively. Example- α –pehnyl alchol and thionyl alchol •It was found that product is formed with complete retention of configuration on the starting alcohol. •The reaction proceeds with the formation of a chlorosulphite ester which collapes with elimination on SO2. •The chlorosulphite ester could form the product by SN2 with inversion, and SN1 with racemisation. MECHANISM OF Sni on the basis of experimental fact First step- formation of chlorosulphite (intermediate). Second step- dissociation of the chlorosulphite ester to form an ion –pair (not dissociated). Because of the geometry of the ion pair, the chlorine atom is forced to attach the carbonium ion from the side as original C-O bond of the alchol (internal attack). If (S)-1-phenyl ethanol and SOCl2 react in presence of a tertiary amine such as pyridine, the product is (R)-1-phenylethylchloride is obtained, i.e. Inversion of configuration occurs. The pyridine reacts with the HCl, produced in the first step of the pyridinium hydrochloric acid and Cl¯ being an effective nucleophile, attacks the alkyl chlorosulphite from the back via SN2 reaction. Thus we can say in prescence of pyridine, reaction follows the SN2 mechanism and withoput it follows the SN1 mechanism. RATE OF REACTION- both reactant plays crucial role in reaction. Therefore, RATE= k[ROH] [ SOCL₂]. Nucleophilic Substitution at an Allylic Carbon Allylic substrates rapidly undergo nucleophilic substitution reactions . usually accompanied by a certain kind of rearrangement known as an allylic rearrangement. When allylic substrates are treated with nucleophiles under SN1 conditions, two products are usually obtained: the normal one and a rearranged one. • ReasonTwo products are formed because an allylic type of carbocation is a resonance Hybrid so that C-1 and C-3 each carry a partial positive charge and both are attacked by nucleophile resulting in the formation of two products: R-CH=CH-CH₂⁺ • R-C⁺H-CH=CH₂ This mechanism has been called the SN1’mechanism. If equilibrium reached stable product (thermodynamically controlled product) Greater amount Not reached less stable product (kinetically controlled product) Greater amount • Nucleophilic substitution at an allylic carbon can also take place by an SN2 mechanism, in which case no allylic rearrangement usually takes place. • In which the nucleophile attacks at the γ carbon rather than the usual position. This mechanism is called SN2’ mechanism and is an allylic substitution. The SN2’ mechanism takes place under SN2 conditions where α substation sterically retards the normal SN2 mechanism. • When a molecule has in an allylic position a nucleofuge (leaving group) capable of giving the SNi reaction, it is possible for the nucleophile to attack at γ the position instead of the a position. This is called the SNi’ mechanism and has been demonstrated on 2buten-1-ol and 3-buten-2-ol, both of which gave 100% allylic rearrangement when treated with thionyl chloride in ether. Ordinary allylic rearrangements (SN1’) or SN2’ mechanisms could not be expected to give 100% rearrangement in both cases. Nucleophilic Substitution at an Aliphatic Trigonal Carbon • The compounds containing a trigonal carbons, especially when the carbon is double bonded to an oxygen, a sulphur, or a nitrogen undergo nucleophilic substitution through tetrahedral mechanism, often reaction follows the second order called addition-elimination. • In tetrahedral mechanism first the nucleophile attacks to give a tetrahedral intermediate and then the leaving group departs. • As expected, this reaction is catalysed by acid because, protonation decreases the electron density at the carbon undergoing substitution, which facilitates the attack of nucleophile: Nucleophilic Substitution at a Vinylic Carbon Concerned with the nucleophilic substitution at unsaturated carbon such as vinylchloride (CH₂=CHCl). Nucleophilic substitution at a vinylic carbon is difficult under normal condition and is extremely slow compared to substitution at saturated carbon. The Vinyl chloride are essentially inert towards nucleophiles. Reason1. Vinyl C-X bond (x= halogen, oxygen, nitogen) is stronger than the alkyl C-X bond because of a resonance interaction between the double bonds and an unsaturated pair on X. This interaction also weakens and polarizes the pi bond, which is why such compounds are reactive towards the electophilic addition. 2. The sn2 transition state as well as sn1 intermediate (a vinyl cation) are too high in energy to be readily accessible. • Thus the rate determining step involves the breaking of C-X bond. The bond in vinyl halide is harder to break and reaction is slow. • After a lot of reserch, • The vinylic cation can readily made through by solvolysis of the SN1 kind if two conditions are met: 1. The leaving group is extremely good one. 2. The vinylic group contains electron releasing substituents. It takes 16-18 kcal more energy to break a C-X bond in vinyl halide than corresponding alkyl halide. Most commonly used for this purpose is the super leaving group – trifluro methanesulfonate (-OSO2CF3) WHICH is known as TRIFLATE. The powerful electron withdrawing F- ATOM( through dispersial of negative charge) help to stabalise the triflate anion, and makes the parent acid CF3SO2OH one of the strongest LOWRY- BRONSTED ACID known, much stronger than known H2SO4 AND HClO4. REFRENCE1. Advanced organic chemistry: reaction mechanisms and structure by Jerry March; McGraw Hill 2.Advanced organic chemistry by Dr. Jagdamba singh and Dr. L.D.S.Yadav; pragati prakshan 3.Organic reactions and their mechanisms ( second edition) by P.S. KALSI 4. REACTION MECHANISM in organic chemistry by S.M. Mukhrjee and S.P. Singh and Macmillan. THANK YOU