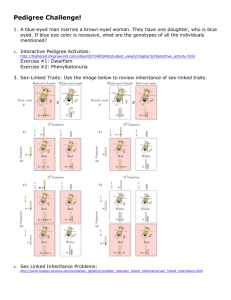
Sex-Linked and Nontraditional Modes of Inheritance Minh Thong Le, PhD. Etiology and genes • Multiple gene disease • Single gene disease (or monogenic): OMIM database, 1960s by Dr. Victor A. McKusick 155 Mb 60 Mb 16.5 kb X CHROMOSOME INACTIVATION • Dosage compensation in sex chromosomes proteins • Lyon’s hypothesis (1960) • Early random inactivation of one Chr. X on each female cell • Random but fixed process • Females = mosaics (50|50) • Males = hemizygous Females = mosaics (50|50) Cell memory maintained by epigenetic mechanisms Cell memory maintained by epigenetic mechanisms Examples: Calico cat Examples: X-linked ocular albinism (melanin defect) Mom Son New findings of X inactivation • Start from day 7 – 10 after fertilization • Initiated at X inactivation center (~ 1 Mb) and expand • Placenta has only Xmale inactivated • Reactivation in female germline stem cell • Barr body number is only one less than number of X chromosomes (Only one X chr. active in each cell == normal dose of protein) • Incomplete inactivation (15% escaped) XXY X inactivation mechanisms • Long noncoding RNA (lncRNA): XIST gene in inactivation center • Late replication • Condensation • Methylation • Histone deacetylation SEX-LINKED INHERITANCE • Y linked • X linked • Recessive • Dominant • But: • Variable expression • Incomplete penetrance • Random X inactivation X-Linked Recessive Inheritance • Complicated, depend on sex of affected peoples and genotypes of the parents • Well known disease: • Hemophilia A • Duchenne muscular dystrophy • Red–green colorblindness Other diseases X-Linked Recessive Inheritance: consequence • Female: • Homozygous of disease gene: expressed disease phenotype • Heterozygous carrier: differ from autosome recessive • 50 % cells normal • 50 % cells expressed disease phenotype • In total, 50 % of normal protein level is sufficient mild or no effect • Male: • Hemizygous of disease gene express the disease X-Linked Recessive Inheritance: frequency • Frequency of disease much different between male and female • Frequency of disease allele q == recurrence of affected male >>> female q2 • Ex: hemophilia A: 1 per 10,000 males • q = 0.0001 • Affected female = q2 = 1/100000000 X linked disease is much more common in male than in female X-Linked Recessive Inheritance: transmitting pattern Common pedigree • No father to son transmission (at least not by father’s gene) • But all daughters receives bad gene from affected dad X-Linked Recessive Inheritance: transmitting pattern Common matings X-Linked Recessive Inheritance: transmitting pattern Less common mating X-Linked Recessive Inheritance: other cases • Female with heterozygous for recessive disease also affected: • X inactivation is random (! Not 50|50) • About 5% of female heterozygotes exhibit hemophilia A and are termed manifesting heterozygotes. • Mildly affected • Case of female express all disease on Chr. X ??? • Chr X – segment rearrangement X-Linked Dominant Inheritance: characteristics • Less prevalence • Twice common in females than males • No father-to-son transmission • Less skipped generations • Ex: • Hypophosphatemic rickets (impaired reabsorb phosphate ==> abnormal bone) • Incontinentia pigmenti type 1 (only in female) • Rett syndrome: MECP2 gene mutation (chr condensasion disorder) X-Linked Dominant Inheritance: transmitting pattern X-Linked Dominant Inheritance vs Recessive Inheritance Y-Linked Inheritance • Very simple to see ^^ • Direct transmitted from father to son • Ex: • Hearing loss by DFNY1 gene SEX-LIMITED AND SEX-INFLUENCED TRAITS: not sex linked • Sex-limited: occurs in only one of the sexes • Due to anatomical differences • Ex: Inherited uterine or testicular defects • Sex influenced: • Not mainly due to the sex chr gene but partly relate (may be elevated the effect) • Ex: male-pattern baldness: both male (more common) and female • Related in part to sex differences in hormone levels • Autosomal genes MITOCHONDRIAL INHERITANCE • Critical important for cell survival • Genome size: 16,569 bp • 2 rRNA • 22 tRNAs • 13 polypeptides relate to oxidative phosphorylation • ~ 1000 nuclear genes have products transfer to mitochondria • Mainly in maternal line (very less and rare in pathernal line) • Mutation rate: 10 time higher than nuclear MITOCHONDRIAL INHERITANCE • Heteroplasmy: portion of mutant mtDNA per normal in each cell (more mutant more effected • Random divided in cell propagation • high variation in effects (diganostics???) • More serious effect in organs need lot of energy (nervous system) • Ex: Leber hereditary optic neuropathy (LHON) (miss sense mutation => optic nerve death) • Other: single-base mutations, deletion, and duplications • late-onset deafness • Some cases of Alzheimer disease • Contribute to aging process MITOCHONDRIAL INHERITANCE: transmitting pattern • Only female to offprings • Complete penetrance (but in variation) GENOMIC IMPRINTING • Mendel’s theory: same phenotype if the mutant allele inherited from mom or dad: But not always true • Many gene in humans are inactived (not transcripted to RNA) from specific parent (other copy is active) • Resulting in the protein dose • ~ 100 human genes are imprinted • Imprinted genes located in gene clusters • Imprinted genes: heavily methylated, histone hypoacetylation, condensation of chromatin == X inactivation in mechanism GENOMIC IMPRINTING Imprinting pattern must be reset during gemetogenesis GENOMIC IMPRINTING: Beckwith–Wiedemann Syndrome Risk of increased kidney tumor Uniparental disomy, in this case affecting chromosome 11 Loss of maternal methylation of IGF2 Beckwith–Wiedemann Syndrome: • 20 – 30 % cases: pathernal uniparent disomy of chr 11 or loss of methylation of DMR1 • Normal: IGF2male active & IGF2female inactive only 1 copy active • Affected: pathernal uniparent disomy 2 active copies increased dose • 50 – 60 % cases: loss of methylation in DMR2