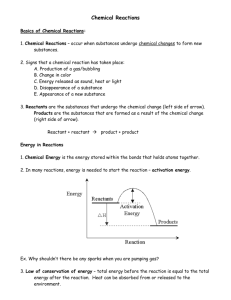
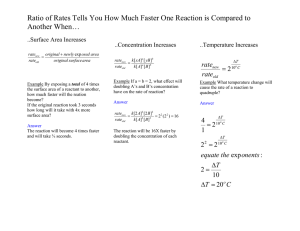
Quiz 1. 50 g of benzene (C6H6) is placed in a container with 160 g of oxygen gas. After the reaction 30 grams of water were collected. What is the percent yield? How much excess reactant was left over after the reaction? 2 C6H6 + 15O2 ---> 6H2O + 12CO2 1. For the following equation determine which reactant is the limiting reactant and which reactant is in excess. The amounts of reagent used are shown. 3Fe + 4H2O Fe3O4 + 4H2 2. Aluminum reacts with oxygen to form aluminum oxide: 4Al + 3O2 ---> 2Al2O3. If 75.0g of Al and 200.0 g of oxygen are reacted, and 75.0 g of aluminum oxide is produced, what is the percent yield for the reaction? 4Al + 3O2 2Al2O3