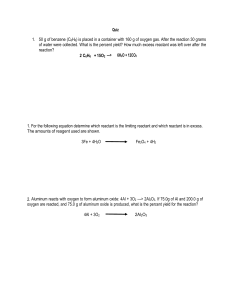
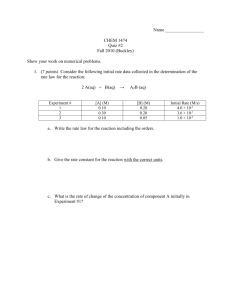
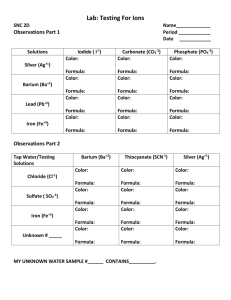
Mass-Mass & Limiting Reactant Practice 1. 300.0 g of hydrogen sulfate is reacted with excess barium oxide. What mass of water will be produced? 2. How much nickel is produced when 575 g of nickel (III) oxide reacts with excess zinc? 3. In the above reaction (#2), how much zinc is required to react with the 575 g of nickel (III) oxide? 4. A reaction between magnesium hydroxide and barium carbonate produces 57.2 g of magnesium carbonate. How much barium hydroxide is made in this reaction? 5. 80.00g of sodium chloride is reacted with 100.00g silver nitrate. How much of each product will produced? (Write the complete equation with the masses under each formula.) 6. In the above reaction determine the mass of the excess reactant that will be left-over in 2 ways: a. Use the Law of Conservation of Mass b. Determine the mass of the excess reactant required to react with the mass of the limiting reactant. 7. Refer back to your reaction in number 5. A student conducted this experiment in chem lab and determined the precipitate in this reaction to be the silver chloride. The student filtered, dried, and massed the precipitate and found they made 96.2 g of silver chloride. What was their percent yield in this experiment? What are some possible explanations for this percent yield?