Uploaded by
Jonah Kristy Desabelle
Properties of Matter: States & Changes - Elementary Science
advertisement

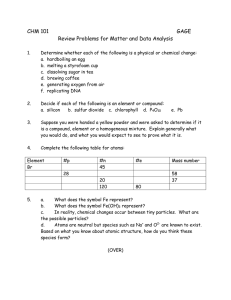
St. Theresa’s College of Cebu College Department Don Ramon Aboitiz St., Cebu, City, 6000, Phils, SCI 200 Teaching Science in the Elementary Grades: Biology & Chemistry Learning Hand out 1 Topic: Properties of Matter Subtopics: Matter, States of Matter, Physical & Chemical Properties (i.e changes) Matter Everything around you is matter. Atoms and compounds are all made of very small parts of matter. Those atoms go on to build the things you see and touch every day. There are only 118 different types of atoms known to man. Frequently, atoms are bonded together to form "molecules". Everything that has mass and volume (occupies space). Mass Volume Mass is the amount of matter in an object. Volume is the amount of space something You might have a small object with a lot of occupies. Words such as big, little, long, or short mass such as a statue made of lead (Pb). You are used to describe volumes. A marble takes up a might have a large object with very little mass small volume while a star occupies a large such as a balloon filled with helium (He). volume. Different states of matter will fill volumes Mass is not the same with weight. Mass is a in different ways. measure of the matter in an object while weight is a measure of gravity’s pull on an object. STATES OF MATTER Matter can be found on Earth in three main forms or states: solids, liquids, and gas. Below are the general definition and characteristics of these states of matter. SOLID LIQUID GAS Solids have definite shape and Liquid has a definite volume but Gas has no definite shape and volume. with no definite shape. no definite volume. There are different kinds of Different liquids have different solid matter and each has its characteristics. They come in own properties. Some are soft different colors, tastes and thickness. like the fabrics and others are hard like steel. Some are brittle like the hard clay, some are malleable like the gold, and some are ductile like the copper wire. Some are strong like wood and iron, others are not, like some plastics. Gases, liquids and solids are all made up of microscopic particles, but the behaviors of these particles differ in the three phases or states. Below are the behaviors of the particles of each state of matter. Prepared by: Ms. Jonah Kristy G. Desabelle, LPT CATEGORY: Volume & its Shape Compression SOLID retains a fixed volume and shape the particles are locked into place ; rigid LIQUID assumes the shape of the part of the container which it occupies the particles can move/slide past one another not easily compressible the particles have little free space between particles flows easily the particles can move or slide past one another GAS assumes the shape and volume of its container the particles can move past one another not easily compressible compressible the particles have little the particles have lots free space between of free space between particles particles does not flow easily flows easily Flow the particles are rigid the particles can move and cannot move or past one another slide past one another PROPERTIES OF MATTER The properties of matter refer to the qualities/attributes that distinguish one sample of matter from another. These properties are generally grouped into two categories: physical or chemical. Organizational breakdown of chemical and physical properties of matter: PHYSICAL PROPERTIES Physical properties can be observed or measured without changing the composition of matter. Physical properties are used to observe and describe matter. Physical properties of materials and systems are often described as intensive and extensive properties. This classification relates to the dependency of the properties upon the size or extent of the system or object in question. An intensive property is a bulk property, meaning that it is a physical property of a system that does not depend on the system size or the amount of material in the system. Examples of intensive properties include temperature, refractive index, density, and hardness of an object. When a diamond is cut, the pieces maintain their intrinsic hardness (until their size reaches a few atoms thick). In contrast, an extensive property is additive for independent, non-interacting subsystems. The property is proportional to the amount of material in the system. Prepared by: Ms. Jonah Kristy G. Desabelle, LPT Intensive properties: A physical property that will be the same regardless of the amount of matter. - density: ρ=mvρ=mv - color: The pigment or shade - conductivity: electricity to flow through the substance - malleability: if a substance can be flattened - luster: how shiny the substance looks Extensive Properties: A physical property that will change if the amount of matter changes. - mass: how much matter in the sample - volume: How much space the sample takes up - length: How long the sample is PHYSICAL CHANGE It refers to the change in which the matter’s physical appearance is altered, but composition remains unchanged. A physical change takes place without any changes in molecular composition. The same element or compound is present before and after the change. The same molecule is present throughout the changes. Physical changes are related to physical properties since some measurements require that changes be made. PHYSICAL CHANGES OF EACH STATE OF MATTER: - Solid is distinguished by a fixed structure. Its shape and volume do not change. In a solid, atoms are tightly packed together in a fixed arrangement. - Liquid is distinguished by its malleable shape (is able to form into the shape of its container), but constant volume. In a liquid, atoms are close together but not in a fixed arrangement. - Gas is made up of atoms that are separate. However, unlike solid & liquid, a gas has no fixed shape and volume. CHEMICAL PROPERTIES Chemical properties of matter describe its "potential" to undergo some chemical change or reaction by virtue of its composition. What elements, electrons, and bonding are present to give the potential for chemical change. For example hydrogen has the potential to ignite and explode given the right conditions. This is a chemical property. Metals in general have they chemical property of reacting with an acid. Zinc reacts with hydrochloric acid to produce hydrogen gas. This is a chemical property. CHEMICAL CHANGE Chemical change results in one or more substances of entirely different composition from the original substances. The elements and/or compounds at the start of the reaction are rearranged into new product compounds or elements. A CHEMICAL CHANGE alters the composition of the original matter. Different elements or compounds are present at the end of the chemical change. The atoms in compounds are rearranged to make new and different compounds. SOURCES: - Balagtas, et.al., 2011. Growing with Science and Health 3. Rex Book Store, Inc., Quezon, City, Philippines., pp.139-146. - Chemistry LibreTexts. Physical and Chemical properties of Matter. Retrieved from https://chem.libretexts.org/Bookshelves/Inorganic_Chemistry/Modules_and_Websites _(Inorganic_Chemistry)/Chemical_Reactions/Properties_of_Matter - States of Matter. Retrieved from https://www.chem.purdue.edu/gchelp/atoms/states.html Prepared by: Ms. Jonah Kristy G. Desabelle, LPT Prepared by: Ms. Jonah Kristy G. Desabelle, LPT