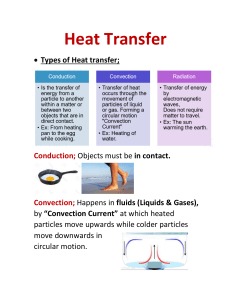
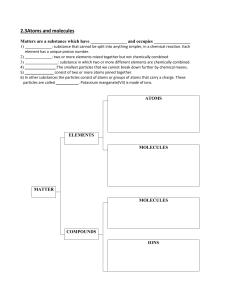
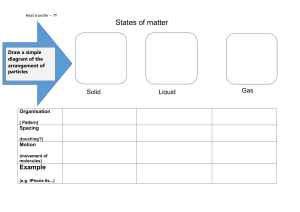
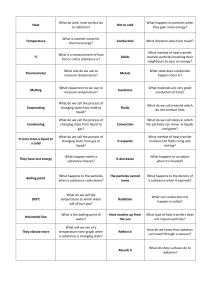
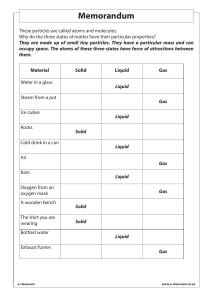
HEAT ENERGY TRANSFER HEAT AND INTERNL ENERGY HEAT is the measure of the total kinetic energy of the atoms and molecules of substance due to its internal energy Proportional to mass Larger mass -- more amount of heat Internal energy The total KE and PE of all atoms and molecules in a material is called the internal energy. The hotter a material, the faster the particles move, and the more internal energy it has. If a hot material is in contact with a cold material, energy is transferred = HEAT. The hot material loses internal energy, the cold material gains internal energy. When an object is heated Measuring the amount of heat energy Heat transfer Conduction Convection Radiation conduction EVAPOURATION High energy particles leave the surface Happens any temperature just below the boiling point Leaves low energy particles