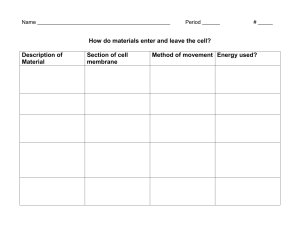
Colligative Properties Now let's go back to a question asked at the very beginning of this lesson, and that is - how are solutions different than pure liquids? One of the ways in which they are different, is that when you add a solute to a liquid both the freezing point and boiling point of the solution change. Water, of course, is the liquid we will be dealing with. The freezing point of pure water is 0°C. The normal boiling point of water is 100°C. But if you make a solution using water as the solvent, the freezing point of that solution will not be 0°C nor will the boiling point be 100°C. In addition the vapor pressure of the liquid changes. Also, something called the osmotic pressure of the liquid changes and that is related to the process of osmosis. In this section we will look at each of these in turn. The freezing point depression, boiling point elevation, vapor pressure lowering, and osmotic pressure are all related to one another, because the magnitude of the change depends on the concentration of solute particles. It is also dependent on the nature of the solvent. These properties are not so much dependent on the nature of, or the chemical properties of the solute that is dissolved, but simply on the number of solute particles present, whether they are ions or molecules doesn't make too much difference except in the number. It is the concentration that make the difference, not the nature. Of course, that concentration does have to take into account whether that solute dissociates and if so, how much. Because of this they are all grouped together as a set of properties, and they are called the colligative properties. Freezing Point Depression | Boiling Point Elevation | Vapor Pressure Change | Osmosis Freezing Point Depression Freezing point depression is not just another way of referring to the early February blues. Instead, it has to do with the change that occurs in the temperature at which a liquid freezes (or a solid melts) when a solute is dissolved in it. For example, consider the effect that salt has on the melting point of ice (which is the same as the freezing point of water). In the Midwest, salting of roads is very common during the winter; it melts the ice and snow that's present on the roads. Here in the Northwest, sanding is more common than salting for roads but some people will use salt on sidewalks. If you've ever made ice cream in a cranked churn, you probably layered ice and rock salt to lower the freezing point enough to freeze the ice cream mixture in the canister. Another example of this is the use of antifreeze in car radiators. By using a solution instead of pure water in the radiator, the liquid will not freeze until you get to some temperature below 0°C (which is 32°F), rather than freezing right at 0°C. The next time you see a container of antifreeze, look on the label and it will show you that as you increase the amount (concentration, actually) of the antifreeze in the car's cooling system the freezing point of the solution decreases. There is a limit to that, and the instructions usually say that you should not exceed a certain percentage. If you get to the point where you have water in the antifreeze rather than antifreeze in the water, the freezing point starts going back up--although it is still very low. In general, for dilute solutions, the amount of change in the freezing point is proportional to the concentration of the solute in the solution. Boiling Point Elevation The effect on the boiling point is just the opposite; that is, the boiling point of a liquid is increased if something is dissolved in it. Again, the antifreeze in a car radiator is an example of this. In the advertisements it's called summer protection against boil-overs. Essentially what they have done is raised the boiling point of the liquid, of the water, by making it a solution. One way of summarizing both freezing point depression and boiling point elevation is to say that the addition of solute extends the temperature range over which the liquid can exist. It will boil at a higher temperature and it will freeze at a lower temperature. Note that as the concentration of the solute increases, so does the effect on both the freezing point and the boiling point. A very practical use of this phenomenon was mentioned earlier - antifreeze. Almost anything can be used to lower the freezing point of water, or to raise the boiling point, as long as it dissolves in water. But there are some very important, practical limitations. For example, if you have a very volatile material such as ethanol, it would not work well in a car's radiator, because as the engine got hot the ethanol could vaporize and leave; it would no longer be dissolved in the solution, therefore it would not be able to lower the freezing point of the solution. On the other hand an ionic material would work but it would probably enhance the corrosion of the interior of the car's engine. You don't want that to happen either. So you have to be very careful about the other physical and chemical properties of the material that you choose when you want to lower the freezing point or raise the boiling point for some particular purpose. In general, the extent of the boiling or freezing point change is proportional to the number of moles of solute added to a certain amount of water, regardless of what specific chemical is used. Electrolyte Effect However, you have to take into account the degree of dissociation of the solute. If a strong electrolyte dissociates into two ions, it will be twice as effective at changing the boiling or freezing point. If a strong electrolyte dissociates into three ions, then it is three times as effective as the same number of moles of a nonelectrolyte. If a weak electrolyte dissociates 5% then it would be 5% more effective than an equal number of moles of a nonelectrolyte. Vapor Pressure Change Another property of a liquid that changes when a solute is added to it, is the vapor pressure. First let's review what vapor pressure is. As you know, liquids will evaporate. The rate and extent to which it evaporates depends on the temperature. If you put a liquid at a certain temperature into a closed, evacuated container it will evaporate until the vapor exerts a certain amount of pressure. That pressure is called the vapor pressure. An evacuated container is not necessary. If the liquid evaporates into air, the vapor is mixed with air and the pressure that it exerts cannot be measured directly. However, the vapor does still exert pressure and it is still called the vapor pressure of the liquid. The amount of evaporation increases when the temperature increases. When the temperature is such that the vapor pressure is equal to the atmospheric pressure, the liquid boils. That temperature is called the boiling point. (This is why cooking times and techniques have to be adjusted for high altitude cooking. Since, at high altitudes, the atmospheric pressure is lower than at sea level, the temperature needed to raise the vapor pressure equal to the atmospheric pressure is lower. A pot of water set to boil pasta, for example, will boil at a temperature lower than 100 oC.) This graph shows the vapor pressure of pure water increasing as the temperature increases. When the temperature reaches 100°C the vapor pressure reaches 1 atm and the water boils. When a solute is added to a liquid, it will decrease the vapor pressure of the liquid, as long as the solute itself is not volatile. In fact this is the way in which the boiling point is affected. Remember, the boiling point is the temperature at which the vapor pressure equals the atmospheric pressure. So if you add something to the solution that decreases the vapor pressure - then you will have to heat the liquid to even higher temperatures before the vapor pressure reaches atmospheric pressure. That's how the boiling point is increased. Osmosis A phenomenon that is related somewhat to the change in freezing point, the change in boiling point, and the change in vapor pressure of solutions when compared to pure solvents, is the process of osmosis. I'm sure you've heard of it. I'd like you to take a look at it now (using the pictures on this pages) and later when you are in the lab. I also recommend that you record your observations in exercise 18 in your workbook. This osmosis apparatus contains a pure water separated from a solution by a thin membrane. So we have three things: a solution, a membrane, and pure solvent. Water is the pure solvent in this case. By looking at the change in liquid levels, you can see that something has moved from the water, through the membrane, into the solution. If something came from pure water it had to be water molecules because pointer marks initial there was nothing position else there. 2 minutes --------------> So the water passed through the membrane from the water side to the solution side. Actually, water passes through the membrane in both directions but it moves faster into the solution than out of it. Water can go in either direction, but the solute particles within the solution cannot pass through the membrane. What seems to happen is that the top of tape marks initial position presence of the solute particles restricts the flow of water molecules from the solution into the pure solvent. But they are not able to restrict the motion of the pure solvent into the solution. Consequently, the water passes from the pure solvent to the solution in greater amounts than water molecules from the solution pass into the pure solvent. So, the water passes through the membrane in both directions, but it passes through at a higher rate from the pure solvent into the solution, than it passes from the solution into the pure solvent. That process is called osmosis. Another aspect of osmosis is something called osmotic pressure. As solvent molecules pass through the membrane into the solution, they build up pressure. If the side with the solution were closed off and had a pressure gauge mounted on it, you would be able to read the osmotic pressure generated by the flow of water into the solution. Here is another way of describing osmotic pressure. The flow of water into the solution can be stopped by applying pressure to the side where the solution is. The amount of pressure needed to stop the flow is the osmotic pressure. Actually the flow of molecules is not stopped, but the flow is equal in both directions and cancels out. It is not necessary for one of the liquids to be pure water in order for osmosis to occur. All that is necessary is for the concentrations on the two sides of the membrane to be different. Isotonic Solutions In the diagram shown here the dotted line represents a semipermeable membrane through which water molecules (but not solute particles) can pass. The small dots represent water molecules and the larger red dots represent solute particles. Note that the solute concentration is the same on both sides of the membrane. The solutions are said to be isotonic compared to one another. Because the solute concentrations are the same on both sides of the membrane, water molecules move through the membrane equally well in both directions. There is no net flow of water in either direction. Hypertonic Solutions In this diagram the solution on the left side of the membrane has a higher solute concentration than the solution on the right side of the membrane. The solution on the left is said to be hypertonic compared to the one on the right. The higher solute concentration on the left reduces the flow of water molecules from left to right, causing a net flow of water from the right to the left. If the right side represented a cell placed in a hypertonic solution, water would leave the cell causing it to dehydrate and collapse. Hypotonic Solutions In this diagram the solution on the left side of the membrane has a lower solute concentration than the solution on the right side of the membrane. The solution on the left is said to be hypotonic compared to the one on the right. The lower solute concentration on the left allows for increased flow of water molecules from left to right, causing a net flow of water from the left to the right. If the right side represented a cell placed in a hypotonic solution, water would enter the cell causing it to swell and perhaps burst.