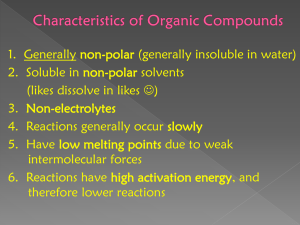
Reactivity of Aliphatic and Aromatic Hydrocarbons Rannilo D. Rocas Jr. Department of Biochemistry, College of Humanities and Sciences, De La Salle Medical and Health Sciences Institute, City of Dasmariňas, Cavite 17 Mar 2020 ABSTRACT Hydrocarbons are compounds composed only of carbon and hydrogen atoms. The first type of hydrocarbon is aliphatic hydrocarbon, which is classified as alkane, alkene, and alkyne. The second type is aromatic hydrocarbon, a circularly structured organic compounds that contain sigma bonds along with delocalized pi electrons. Saturated hydrocarbons contain only single bonds between carbon atom, while unsaturated hydrocarbons contain double bonds or triple bonds. Unsaturated hydrocarbon is more reactive since it contains pi electrons which are nucleophilic in nature and thus more reactive. In this experiment, the reactivity of following compounds we’re tested: petroleum ether, cyclohexane, cyclohexene, xylene, hexane, and petrolatum. Amongst these compounds, the cyclohexene is the most reactive, while the other compounds didn’t react with the reagents. It reacted with KMnO4, with a color change from purple to brown, due to the presence of MnO2. In bromine test, the color of the solution became clear, indicating that the reaction proceeded. Cyclohexene when added in sulfuric acid, resulted in a single layered solution, since the product of the reaction was soluble in sulfuric acid. All the hydrocarbons are nonpolar, hence became soluble only in nonpolar solvent (hexene). Hydrocarbons reacted with oxygen and underwent combustion reaction. Keywords: Hydrocarbons, Reactivity, Saturated, Unsaturated, Aliphatic, Aromatic, Solubility, Combustion. Introduction: Hydrocarbons are the simplest organic compounds, composed only of carbon and hydrogen. It is also separated into two types, namely aliphatic hydrocarbons and aromatic hydrocarbons. There are three types of aliphatic hydrocarbons, wherein each type was characterized by the number of covalent bonds being formed. Alkanes are aliphatic hydrocarbon with single covalent bond, alkene having double covalent bond, and alkyne having triple covalent bond. Aliphatic hydrocarbons also form rings which is called cycloalkane, cycloalkene, and cycloalkyne. Aromatic hydrocarbons are ring systems which contains overlapping p orbitals. The condition of the electrons in the aromatic compound gives a physical and chemical properties that are different from alkanes. The aromatic word was coined to the compound since these compounds were particularly fragrant. However, the term aromatic in modern chemistry denotes that these compounds has a very stable ring (Ball, 2014). Figure 1: Classification of hydrocarbons Unsaturated compounds contain a double or triple bond, which enables these compounds to be more reactive. A saturated organic compound has only single or bonds between carbon atoms and are less reactive than unsaturated compounds (Smith, 2007). Some reactions that unsaturated hydrocarbons partake in are oxidation by the use of KMnO4, bromination by Br2, and addition reaction by H2SO4 Aromatic compounds and alkanes are not as reactive as alkenes and alkynes. 2 Alkenes react with potassium permanganate solution undergo oxidation reaction with alkenes. The dilute KMnO4 solution has a deep purple color; no reaction would result in retention of the color. When it reacts with unsaturated aliphatics it produces MnO2, a brown precipitate. carbon dioxide and water. Figure 5: Combustion of hydrocarbons Objectives: 1. Differentiate the two types of hydrocarbons by their properties. Figure 2: Reaction with KMnO4 Unsaturated hydrocarbons react rapidly with bromine. The reaction is the addition of the elements of bromine to the carbons-carbon double bond. Alkenes, but not alkanes or aromatic hydrocarbons will react with Br2 in solution to produce the corresponding alkyl bromide (or dibromoalkane). The decoloration of the bromine indicates that the reaction proceeds. (Clark, 2019). 2. Classify the hydrocarbons according to their functional group. 3. Predict the reactivity of hydrocarbons. Methodology: A. Solubility of Hydrocarbons On a clean separate test tubes, two drops of each of the following were transferred; petroleum ether, cyclohexane, cyclohexene, and xylene. Three drops of deionized water were then added, the test tubes were shaken up and the observed color was recorded. The same procedure was repeated using a different solvent (hexane). The miscibility of the compounds were recorded. Figure 3: Reaction with Br2 Alkenes react with concentrated sulfuric acid in the cold to produce alkyl hydrogensulphates. Saturated hydrocarbons are unreactive and aromatic compounds also are unreactive because addition reactions are difficult due to resonance stabilization. B. Baeyer’s test On a clean separate test tubes, two drops of each of the following were transferred; petroleum ether, cyclohexane, cyclohexene, and xylene and deionized water (blank). One drop of Baeyer’s Reagent was added to each test tube. The test tubes were shaken up and the colors observed were recorded. C. Reaction with Bromine Figure 4: Reaction of Cycloalkene with sulfuric acid Almost all hydrocarbons will undergo combustion, this occurs when hydrocarbon reacts with oxygen to produce On a clean separate test tubes, two drops of each of the following were transferred; petroleum ether, cyclohexane, cyclohexene, and xylene and deionized water (blank). One drop of Bromine water was added to each test tube. The test tubes were shaken up and the colors of each were recorded. 3 D. Reaction with Sulfuric Acid On a clean separate test tubes, two drops of each of the following were transferred: petroleum ether, cyclohexane, cyclohexene, and xylene and deionized water (blank). One drop of conc. Sulfuric acid was added to each test tube. The test tubes were shaken up. The number of layers and the colors of each were recorded. E. Combustion Figure 6: Observed layers Table 2. Baeyer’s Test In an evaporating dish, two drops of petroleum ether were placed, and then was ignited under the fume hood. The same procedure was repeated using cyclohexane, cyclohexene, and xylene. F. Generation and Analysis of Acetylene A pinch of calcium carbide was transferred in a dry small vial. A syringe was used to add 1 mL of tap water. A sufficient amount of gas was then collected using a syringe. In a separate well of a spot plate, two drops each of Baeyer’s reagent and bromine water was added. The gas was injected on each well, and the reaction was observed for any changes in color or bubble formation. G. Analysis of Petrolatum A small amount of petrolatum was placed on a spot plate, then all the tests were conducted. Results and Discussions: Table 1. Solubility of Hydrocarbons Hydrocarbon Water Hexane Petroleum ether Cyclohexane Immiscible Miscible Immiscible Miscible Cyclohexene Immiscible Miscible Xylene Immiscible Miscible Petrolatum Immiscible Miscible All the compounds were observed to be insoluble in water, since these compounds were nonpolar, and water is a polar solvent, and like dissolves like. When added in a nonpolar solvent which was hexane, all the compounds were observed to be soluble. Hydrocarbon Color Petroleum ether Cyclohexane Purple Cyclohexene Brown Xylene Purple Petrolatum Purple Acetylene Purple Deionized water Purple Purple In using the Baeyer’s test, cyclohexene turns into color brown, while the rest of the compounds didn’t exhibit a color change. Potassium permanganate can only react with unsaturated hydrocarbons, and the color change from purple to brown color indicates that the reaction takes place since the brown color is the indication of a presence of MnO2, which is the product of the reaction along with diol. Figure 7: Observed color change Table 3. Reaction with Bromine Hydrocarbon Color Change Petroleum ether Cyclohexane Clear Cyclohexene Clear Xylene Cloudy (white) Clear 4 Petrolatum yellow Cyclohexene Orange Acetylene Lighter yellow Xylene Orange Deionized water Yellow Petrolatum Non-combustible Petroleum ether, cyclohexane, and cyclohexene reacted with bromine based on the color change observed, however, according to previous experiments, and expected results, cyclohexane wouldn’t react with bromine. Only the presence of available electron pair could cause reaction with bromine, cyclohexene is the only compound that readily has these electron pairs, the disappearance of the yellow color indicates that the bromine was used up and was bonded to the organic compound. All the compounds underwent combustion except with the petrolatum, this might be caused by the physical characteristic of the petrolatum, like having a gel like property, unlike the other hydrocarbons which is in liquid form. Figure 9: Combustion under the fume hood Conclusion: Figure 8: Observed color change Table 4. Reaction with Sulfuric Acid Hydrocarbon Observation Petroleum ether Cyclohexane 2 layers Cyclohexene no layer Xylene 2 layers Petrolatum 2 layers Deionized water 2 layers 2 layers All the compounds were observed to have 2 layers except cyclohexene. Since alkenes and cycloalkenes are the only hydrocarbons that can have addition reaction when combined with sulfuric acid. The product formed from this reaction is soluble in sulfuric acid, thus resulting in a single layered liquid. Table 5. Combustion Hydrocarbon Observation Petroleum ether Cyclohexane Orange Orange Hydrocarbons, being compounds that only contain the hydrogen and the carbon elements, exhibit lesser reactivity compared to other organic compounds, these particular hydrocarbons are saturated and aromatic compounds. Unsaturated hydrocarbons are more reactive when compared to saturated hydrocarbons as in the unsaturated compounds we have double bonds (pi bonds) which are weak and can be easily cleaved by incoming group therefore increasing the reactivity. In saturated hydrocarbons only sigma bonds are present, these bonds are very strong and stable. That principle strongly supports and validates the results of the experiment. Although aromatic compounds also contain pi bonds, it was still not as reactive as alkenes due to resonance, this causes stabilization of the organic compound. Cyclohexene, a pi bond containing hydrocarbon was the most reactive compound amongst the hydrocarbons tested in the experiment. It is recommended that proper labeling of the test tubes must be done in order to avoid misinterpretations of results, since all the hydrocarbons have the same physical appearance. In testing the solubility, a few drops of the solvent can be added for a better visualization of the presence of double layer in the test tube. Familiarizations of the properties of different types of hydrocarbons could also be helpful, having this background would allow for expected results, thus any results that has an error could easily be identified and addressed. 5 References: Ball, D. W., & Key, J. A. (2014, September 16). Hydrocarbons. Retrieved March 17, 2020, from https://opentextbc.ca/introductorychemistry/chapter/hydro carbons-2/ Bonifacio, M. C. (2020). Organic Chemistry Laboratory Experiments (2020th ed.). Libretexts. (2019, June 5). Reactions of Alkenes with Bromine. Retrieved March 15, 2020, from https://chem.libretexts.org/Bookshelves/Organic_Chemistry /Supplemental_Modules_(Organic_Chemistry)/Reactions/A ddition_Reactions/Electrophilic_Addition_Reactions/Reacti ons_of_Alkenes_with_Bromine Properties of Hydrocarbons. (n.d.). Retrieved March 17, 2020, from http://www.mendelset.com/articles/689/properties _hydrocarbons Smith, Michael B.; March, Jerry (2007), Advanced Organic Chemistry: Reactions, Mechanisms, and Structure (6th ed.), New York: Wiley-Interscience, ISBN 978-0-471-72091-1