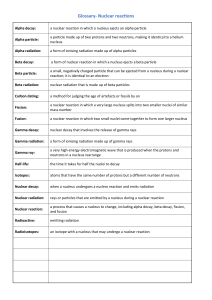
Advanced Chemistry Name___________________________ NOTES Ch. 16: Nuclear Chemistry What IS it? I. Radioactive Decay A. When an _____________ nucleus loses energy by emitting ______________ B. Radiation: _________________________________________________________________________________ _________________________________________________________________________________ II. Radioactive Decay A. Why are some nuclei unstable? 1. Unfavorable ratio of ______________ to _______________. 2. Nucleus adjusts itself by losing _______________ and emitting ________________. 3. Process known as radioactive decay. 4. An isotope of an element that emits radiation is a called a ______________________. Nuclear Decay & Transmutation Reactions: Description Alpha Type of Particle Example Effects Description Type of Particle Example Effects Description Type of Particle Example Effects Beta Gamma REMEMBER: In a nuclear equation, the sum of the mass numbers and the sum of the atomic numbers on one side of the arrow must equal the sum of the mass numbers and the sum of the atomic numbers on the other side. Radioactive nucleus new nucleus + radiation (α, β, β+, γ)