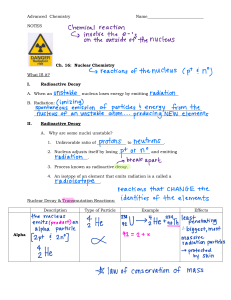
Acknowledgment I would like to express my special thanks of gratitude to my school ( Birla public School, Doha- Qatar) as well as our principal _______who gave me the golden opportunity to do this wonderful chemistry project on nuclear chemistry, which also helped me in doing a lot of Research and I came to know about so many new things. I am really thankful to them. Secondly, I would also like to thank my chemistry teacher ________ who helped me a lot in finalizing this project within the limited time frame. He gave me all the important information regarding the topic and helped me to understand the in-depth of the topic. Lastly, I would like to thank the laboratory assistants and the lab technician to help me conduct the experiments required by the topic for the project, and thank them for all the support and guidance while conducting the experiments, their wisdom and understanding of the lab equipment helped me to take precautions during the experiments and I could successfully complete the experiments on time. INDEX Serial Number Topic Page Number 1. Whats is Nuclear Chemistry 4 2. Sources of radioactive elements 5 -Primordial -Cosmogenic -Anthropogenic 3. Radiation and Nuclear Reactions 6 -Alpha -Beta -Gamma 4. Half Life 9 5. Stimulated Nuclear Reactions 11 -Nuclear fission -Nuclear fusion 6. Conclusion 14 7. Bibliography 15 What is Nuclear Chemistry? Chemical reactions happen when an atom’s outermost electrons undergo changes and the proton and neutrons and even the inner electron shells are usually completely unaffected. When protons and neutrons are directly involved in reactions, huge amounts of energy can be released. Far more than by the transfer of electrons that occur in simple chemical reactions. When changes happen to the nucleus of an atom we rather logically call their study: Nuclear Chemistry. All of these changes involve massive changes in energy. Energy changes which are hundreds of thousands of times larger than in a typical chemical reaction. Because these energy changes are so big we actually get a measurable change in the mass between the products and the reactants. Sources of Radioactive Elements There are essentially three sources of radioactive elements: 1. PRIMORDIAL NUCLIDES Primordial Nuclides commonly known as primordial Nuclides are one of the first atomic species which ever came into existence. The term is quite self-explanatory as the word ‘Nuclides’ means an atomic species which is identified by a specific composition of the nucleus whereas the word ‘primordial’ means ‘primeval ’. These are presumed to have been formed after the Big Bang due to nucleosynthesis between stars and supernovae. Nucleosynthesis is the process of creation of new atomic nuclei from already pre-existing nuclei. Since these Nuclides came into existence billions of years ago and yet have survived, they are said to be highly stable. There are only 286 such Nuclides present All of the 286 known primordial Nuclides have an exceptional half life which is quite significant due to their existence. Cadmium, tellurium, xenon, neodymium, samarium and uranium each have two primordial radioisotopes. 2. COSMOGENIC NUCLIDES Cosmogenic nuclides are atoms that are constantly being amalgamated from the collision of planetary surfaces by cosmic particles or rays (primarily protons ejected from the Sun). 3. ANTHROPOGENIC NUCLIDES The last source of radioactive nuclides is called anthropogenic and is formed from human activity in the formatiotion of nuclear power, nuclear weapons, or through the use of particle accelerators. RADIATION AND NUCLEAR REACTIONS In 1902, Soddy proposed the idea that "radioactivity is the result of a natural change of an isotope of 1 element into an isotope of a different element." Nuclear reactions involve changes in particles in an atom's nucleus and thus cause a change within the atom itself. All elements that weigh more than bismuth (and some lighter) showcase a tendency of radioactivity and thus can "decay" into lighter elements. Unlike standard chemical reactions that combine to form molecules, nuclear reactions end in the transmutation of 1 element into a different isotope or a different element as a whole (remember that the number of protons in an atom defines the element, so a distortion in the number of protons leads to a change within the atom). There are three general types of radioactive decay each named for exactly what is released from the nucleus as it decays. 1. Alpha Radiation (α) Alpha Radiation is the releasing of an alpha particle from a particle’s nucleus. An α particle contains 2 protons and two neutrons As an example let us take uranium. By far the most found naturally obtained form of uranium is the isotope uranium-238. U-238 spontaneously decays into thorium-234, in a process that emits an alpha particle. This is called alpha decay and the atom that it emits is basically a helium nucleus. alpha particles have relatively lower energy, they are pretty heavy as atoms go. alpha particles can be stopped by nothing more than a sheet of paper or cloth. 2. Beta Radiation (β) The second type of radioactive decay is beta decay, which simply emits electrons from an atomic nucleus, transforming the original nuclide to an isobar. Beta decay of a neutron transforms it into a proton by the emission of an electron . It has a higher energy than alpha radiation. The thorium-234 that formed when uranium underwent alpha decay can continue to decay on its own, and when it does, it undergoes beta decay. Releasing an electron and an atom of xenon. . 3. Gamma Radiation (γ) The third type of decay is a little different, because it only emits energy, not a particle. It's called gamma decay, and it releases electromagnetic radiation similar to visible light, or UV radiation, but higher on the energy scale. Because it's just energy, gamma radiation has no mass and contains no protons, neutrons, or electrons. This form of radiation is often released when electrons transition from an unstable excited state, to a more stable state that has a lower energy. That's called the ground state. Depending on how much energy the electron loses, the extra energy can be released in the form of visible, or ultraviolet light, x-rays, or gamma waves. Take the example of nickel-60. Assume there's an atom of nickel-60 with one or more of its electrons in an excited state. Atoms can get to this state when they are themselves the products of radioactive decay, or if they get bombarded with radiation from other reactions pushing their electrons into a higher energy level. Now when all those electrons drop down to the ground state, that atom is going to release some gamma radiation. This kind of transition can also take place where other kinds of nuclear reactions are going on. Half Life Half life is basically the time it takes for exactly one half of the sample to decay. Half lives vary with different nuclei. We can calculate how much of a sample will be subjected to decay by knowing the half life of that sample. When there is a more stable state, a higher energy nucleus undergoes radioactive decay to attain it. This difference in energy is released as what's called ionizing radiation. It's termed “ionising” because it has sufficient energy to add or remove electrons from other atoms, essentially creating ions. As shown, the reaction proceeds in halves, with half of whatever is left of the radioactive element decaying every half-life period. The amount of radioactive isotope remaining after a given number of half-lives can be calculated using the following formula : The decay reaction and T½ of a substance are specific to the isotope of the element undergoing radioactive decay. STIMULATED NUCLEAR REACTIONS Although elements undergo radioactive decay naturally, nuclear reactions can also be stimulated artificially. Although these reactions also occur naturally, we are most familiar with them as stimulated reactions. There are two such types of nuclear reactions: 1. NUCLEAR FISSION The reaction in which a large nucleus splits into two lighter ones and releases a large amount of energy as a side product is termed as fission. The products of the reaction are more stable than the reactants and this is what drives the reaction. A nuclear fission reaction is almost always triggered by “firing” a neutron at an element which splits it into two(or more) lighter elements, during which the bond energy of the parent atom is released mainly as the kinetic energy of the escaping particles which is transmitted to the surrounding as heat. The Fission Reaction of Uranium-235 During the fission of uranium-235, two daughter products and three free neutrons are released. These free neutrons can stimulate the fission of a nearby uranium-235 atom if it collides with it and can start a self-sustaining nuclear chain reaction This chain reaction is the principle of nuclear power. More and more atoms continue to split, releasing a significant amount of energy which can be harvested and used to produce electricity. 2. NUCLEAR FUSION Fusion reactions are very different from fission, it's pretty much the opposite. For one thing, the energy released in a fusion reaction dwarfs the energy output of fission reactions. A common example we see everyday is the work done by our sun. The reactions that drive our sun are like most fission reactions, in which two light nuclei join together to form a heavier one. Hydrogen molecules are fused into helium and heavier elements inside of stars. A lot of energy is released during this in the form of heat and gamma radiations. CONCLUSION This report hopes to provide holistic development in nuclear chemistry for its readers as it did for me. In addition to that, it also educates about its types and consequences of the precarious reaction. Finally it also ultimately serves as a hope for future generations to solve problems never fathomed to exist and strives to make minds wander to the possibilities of its applications after understanding the basic principles and mechanism behind the phenomenon. BIBLIOGRAPHY 1. https://www.encyclopedia.com/science/news-wires-white-papers-andbooks/nuclear-chemistry 2. https://en.m.wikipedia.org/wiki/Primordial_nuclide 3. https://www.visionlearning.com/en/library/Chemistry/1/NuclearChemistry/59 4. https://www.scribd.com/document/295222026/EOCQ-ans-16 5. https://www.youtube.com/watch?v=I0IgOfsyrRA 6. https://www.nr8.com/narratives/5f1fb03a3c038dd8607aea4c