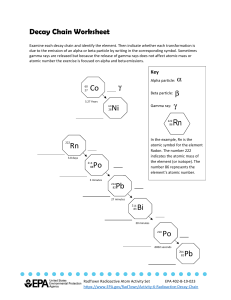
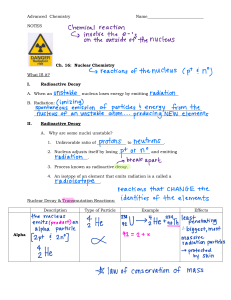
1 ATOMIC PHYSICS RADIOACTIVITY: It is the disintegration (breaking apart) of an unstable atomic nucleus. Radioactivity is random, i.e. it can occur at any time releasing any number of emissions. Three types of emissions are produced; alpha particle (α), beta particle (β) and gamma rays (γ). Examples of Radioactive materials Uranium – 238 Carbon -14 Cobalt -60 Thorium -232 Plutonium-244 Potasium-40 Background Radiation Various rocks in the earth, including granite contain small percentages of radioactive uranium, Thorium and Potassium compounds. Note; Uranium, Thorium and Potassium are the only three naturally occurring radioactive materials. Our bodies also contain traces (small amounts) of radioactive materials as do the bricks and other building materials that are used to build our homes, schools and work places. In addition to these sources we are also exposed to gamma radiation from the sun. These sources of natural radiation constitute the background radiation. We are exposed to background radiation all the time, because it is very low it causes no risk to our health. Dangers of Radioactivity Beta and gamma rays can easily pass through the skin and can damage or even kill cells. They can cause mutations in a cell’s DNA which may lead to cancer. Extremely large doses of radiation can cause radiation burns. Safe HANDLING of radioactive substances Radioactive materials are lifted with forceps or long tongs. In industry they are handled by mechanical tongs operated by remote control equipment from behind thick walls made of lead. They are stored or transported in lead or concrete walls. Workers wear radiation doze badges to check on the amount of radiation they are exposed to. 2 NATURE OF EMISSIONS (i) Alpha Particle It consists of two neutrons and two protons but no electrons, therefore it is a helium nucleus 2+ ( ). The helium nucleus releases two electrons to become an alpha particle. (ii) Beta Particle These are streams of high energy electrons ( ). (iii) Gamma rays These are electromagnetic waves, with wavelength much shorter than that of light. PENETRATION AND IONISATION POWERS Penetration powers are due to the way they interact with matter and ionization occurs when the emissions knock off electrons from atoms (making ions). Alpha (α) particles are stopped by a thin sheet of paper or even 5cm of air at normal atmospheric pressure, so they are the least penetrating of the three. Since they are large, carry two positive charges and slow moving, they are the strongest ionizers. Beta (β) particles are more penetrating than alpha particles, they are blocked by 3 mm of aluminum sheet and go up to 1 m of air at normal atmospheric pressure. Since they carry less charge and move faster than alpha particles, they are less ionizing. Gamma (γ) rays are the most penetrating. They can be reduced but not stopped by lead. Since they are the fastest and carry no charge, they are the least ionizers. IONISATION EFFECTS Ionizing emissions damages the complex chemicals necessary for the functioning of the cell. If this damage is significant, the cell will die. For radiation outside the body, gamma radiation is the most hazardous because of its high penetration so it can reach the interior of the body, whereas the highly ionizing alpha emission will be stopped by the dead skin cells, so it will cause little or no harm to the living cells beneath the skin. For radiation inside the body i.e. if swallowed by mistake or breathed in. The highly ionizing alpha particle will cause more harm than gamma rays. 3 EFFECTS OF ELECTRIC FIELD ON EMISSIONS EFFECTS OF MAGNETIC FIELD ON EMISSIONS METHOD OF DETECTION Geiger- Müller (GM) tube is an instrument specially designed to detect the radioactive radiations i.e. (alpha beta and gamma). It operates on the basis of ionization effect of the radiations If an alpha particle enters the tube, it ionizes the gas inside. This sets a high voltage spark across the gas and a pulse of current in the circuit flows. A beta particle or burst of gamma radiation has the same effect. When the radiation from a radioactive source is measured, the reading always includes any background radiation. So an average for the background alone must be found and subtracted from the total. 4 NUCLEAR REACTIONS Radioactive Decay: The decay is exponential and has a constant half-life. The rate of decay depends on the number of undecayed nuclei at any particular time. ALPHA DECAY This occurs when the nucleus ejects two protons and two neutrons thus reducing its nucleon number (mass no) by four and its proton number by two. +α e.g. Uranium-238 decays into Thorium-234 by the emission of an alpha particle + BETA DECAY This occurs when a neutron decays into a proton and an electron which is emitted at a high speed. The proton number increases by one and the mass number remains the same. +β e.g. Carbon -14 undergoes beta decay + GAMMA DECAY This normally occurs after an alpha or beta decay when a nucleus is left in an excited state (with excess energy). To be more stable the nucleus will lose the excess energy by emitting the γ- rays. The element remains unchanged. + Energy DECAY CURVES Half-life: It is the time taken for half of the radioactive atoms to decay or time taken for activity (count rate) of a radioactive source to decrease by half. 5 Generally: N = No ( )n Where N is No of undecayed nuclei No is Original No of nuclei n is number of half-lifes n= Where n is number of haif-lifes t is time of decay is half-life Example: A radioactive source of mass 200g has a half-life of 8 minutes. Determine the remaining mass after 24 minutes. N = No ( )n n= N = 200 ( )3 = =25g =3 6 USES OF RADIOACTIVITY (i) TRACERS: Radioisotopes can be detected in very small (safe) quantities, so they can be used as tracers. Their movements can be tracked or traced.e.g. checking the function of body organs, tracking a plant’s uptake of fertilizer from roots to leaves or detects leaks in underground pipes. (ii) RADIOTHERAPY: Used to treat cancer. (iii) RADIOCARBON DATING: To determine the age of a sample. (iv) NUCLEAR POWER STATIONS: Used to produce electricity. (v) STERILISING: Medical equiptments such as needles, syringes and dressings are sterilized by a radioactive source. (vi) CONTROLLING POPULATION OF PESTS: They are sterilized. NUCLEAR FUSION: This is the joining of two light nuclei to form a heavier nucleus. e.g. The fusion of two heavier isotopes of hydrogen deuterium ( ( ) and tritium ( ) to obtain Helium ) + neutron + + NUCLEAR FISSION: The fission involves splitting of a heavier nucleus into two, more or less equal fragments. E.g. + + + 2 If the fission neutrons split other Uranium 235 nuclei, a chain reaction is set up. The nuclear fission equation above shows that the number of nucleons is conserved. i.e. The nucleon number adds up to 236 on both sides of the equation, and the proton number adds up to 92 on both sides of the equation. But an accurate adding up of the total mass on each side of the equation would show that there is a small loss of mass on the right- hand side. This lost mass is converted into energy. In 1905 Albert Einstein worked out the relation between mass and energy in his now famous formula. E = mC2 The energy E released in Joules can be found if the mass loss m is given in kilograms and C = 3 x 108 m/s, the speed of light.