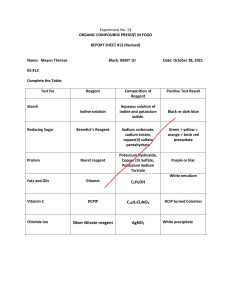
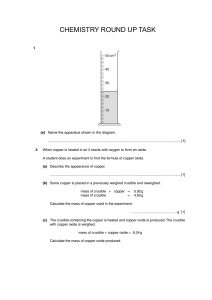
SEKOLAH GLOBAL INDO-ASIA WORKSHEET/ASSIGNMENT 2020 – 2021 Name : Class : Subject : Stoichiometry #2 Date : Score Answer the questions. Submit it by the end of period in the dropbox given. Total mark is 8 1. 2.4 g of Magnesium reacts with 6 g of ethanoic acid. a. Calculate the mass of magnesium ethanoate obtained (2) b. Which is the limiting reagent. (2) c. Which reagent is in excess and how much is it after reaction? (2) 2. 2.5 g Copper (II) oxide reacts with excess sulfuric acid. Calculate the mass of CuSO4 formed. (2) Teacher’s Signature