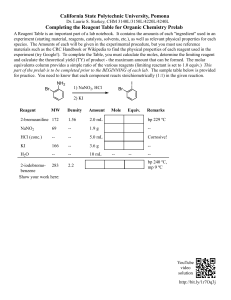
SEARCH FOR THE LIMITING REAGENT IN A CHEMICAL TRANSFORMATION ●ACTIVITY 1: THE MISSING INGREDIENT A pizza maker wants to predict the number of pizzas he can prepare with his stock. His recipe is always the same and for 10 pizzas he needs: - 2,00 kg of dough, - 1,00 L of tomato sauce - and 1,50 kg of cheese. He has in his stock 10 kg of pizza dough, 7 L of tomato sauce and 3 kg of cheese. - How many pizzas can he make? (How much pizza can he make? You will ask your English professor : I couldn’t chose …) - What will be left in the stock after the pizza making? ●ACTIVITY 2: IDENTIFICATION OF THE LIMITING REAGENT This experiment must be done as quickly as possible, so you don’t need to measure the quantities with the best precision. In a test tube, 1mL corresponds to about 1cm in height. The chemical reaction between a hydrochloric acid solution containing 1mol of H+ ions per litre and a sodium hydroxide solution containing 1 mol of OH- ions per litre is studied. The equation of the chemical reaction is: H+(aq) + OH-(aq) → H2O(l) For each chemical system, predict the remaining reagent at the end of the transformation, the limiting reagent that disappears completely and the color taken by the BT. Fill in the table with the forecasts. Then, perform the experiments to check your forecasts. One tube is enough by adding the acid (H+) in three successive manipulations. Correct the errors in red, if necessary. Thymol blue (TB) is an acid-base indicator. An aqueous solution of TB undergoes the color changes from yellow in an acid solution to blue in an alkaline solution. *Chemical system solution OHSolution H+ Remaining reagent Limiting reagent BT color 1 2mL 1mL 2 2mL 2mL 3 2mL 4mL ●ACTIVITY 3: CHEMICAL TRANSFORMATION AND LIMITING REAGENT. Protocol: The chemical transformation is carried out when sodium hydrogen carbonate powder (NaHCO3) is mixed with a solution of hydrochloric acid (H+(aq) + Cl-(aq)) containing 1mol of H+ ions per litre. →Step 1: Filling a graduated burette with the hydrochloric acid solution 1) Empty the water into a "garbage" pot and throw it in the sink. 2) Rinse the burette by pouring a little acid into it. The acid is recovered in the "trash" jar. 3) Turn the tap to close the burette; 4) Fill the burette it with acid 2 cm above zero. 5) Quickly open and close the burette tap to expel the air bubble in the bottom of the burette. 6) Open gently, drop the acid drop by drop to set the level (lower meniscus) to zero. →Step 1) 2) 3) 4) 2: Start of the chemical reaction In an Erlenmeyer, place precisely 1g of hydrogen carbonate powder. With the burette, pour 4 mL of acid. Shake and wait for the effervescence to end. Pour 3 drops of Thymol Blue (BT) →Step 3: Analysis In the reactants the ions Na+, HCO3-, H+ and Cl-. This chemical transformation produces a gas and also produces water. Note: When the produced gas is bubbled, it clouds the lime water. What gas is it? Write the equation of the reaction: →Step 4: Among HCO3- and H+, which is the limiting reagent and the excess reagent? For different volumes of acid, determine the limiting reagent and the excess one and fill in the table. Each time, we add just the volume needed to reach the next system and we have to wait until the end of the effervescence before moving on to the next system. Simply add the missing volume of acid to change the system. 1 Chemical system (Done in step 2 3 2) NaHCO3 1g 1g 1g 4mL 8mL 12mL Acid (H+ + Cl-) (so addition of (so addition of 4mL) 4mL) BT color Limiting reagent Excess reagent 4 1g 16mL (so addition of 4mL)