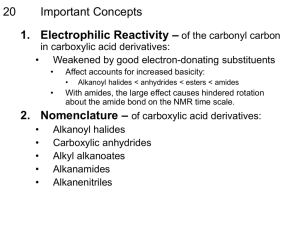
Carboxylic Acid Amides, Esters, and Anhydrides • These derivatives are formed by a condensation reaction with carboxylic acid in which two molecules combine into one while a small molecule is lost (water in this case). Description Amides • General formula of RCONR2 • Named by replacing –oic acid with –amide o Alkyl substituents on the nitrogen atom are listed as prefixes and their location is specified by the letter N• Synthesized by reaction of other carboxylic acid derivatives with either ammonia or an amine. Loss of hydrogen is required for this reaction to take place. o Only primary and secondary amines will undergo this reaction • Lactams: Cyclic amides and are named according to the carbon atom that is bonded to the nitrogen. • May or may not participate in hydrogen bonding. Esters • Dehydration synthesis products of other carboxylic acid derivatives and alcohols. • Esterification Group: substituent that is bonded to the oxygen • Named by placing the esterifying group as a prefix and the –oate suffix replaces -oic acid • Fischer Esterification: mixing carboxylic acids and alcohols under acidic conditions so that they condense into esters. • Lactones: Cyclic esters and named similarly to lactones, but with name of precursor acid molecule included. • • Lack hydrogen bonding, so boiling points are lower than related carboxylic acids. Triacylglycerol: storage forms of fats in the body o Esters of long chain carboxylic acids and glycerol o Can undergo Saponification to produce soap salts. Anhydrides • Also called Acid anhydrides and are the condensation dimers of carboxylic acids which have the general formula RC(O)OC(O)R • Symmetrical anhydrides are named by substituting anhydride for acid in a carboxylic acid. • If asymmetrical, name two chains alphabetically and then followed by anhydride. • Synthesized by the condensation reaction between two carboxylic acids and one molecule of water is lost in condensation. o Hydroxyl group of one acts as a nucleophile and attach the carbonyl group on the other. • • Cyclic anhydrides can be formed by heating the carboxylic acids o Reaction is driven forward by the increased stability of the newly formed rings o Only anhydrides with five or six membered rings are made easily. Have higher boiling points than corresponding carboxylic acids since they are much heavier. Reactivity Principles Relative Reactivity of Derivatives • Reactivity of the carbonyl is determined by the substituents o Anhydrides are most reactive since their resonance stability and three electron withdrawing oxygen atoms are the most electrophilic and thus reactive. o Esters are second most reactive since they contain one less oxygen atom. o Amides have an electron donating amine group and are thus the least reactive Steric Effects • • Steric hindrance is when a reaction does not proceed due to the size of its substituents. Can be seen as a detrimental effect since reactivity is decreased • • Also can be positive since it can help force a certain type of reaction or help in the generation of protecting groups. In general, the size and substitution of the leaving group affects the ability of the nucleophile to access the carbonyl carbon Electronic Effects • • Induction: the distribution of charge across sigma bonds o Differing electronegativity’s between two molecules of a sigma bond cause one end to be positive and the other to be slightly negative. o Effect is relatively weak and gets increasingly weaker as you move farther away from the more electronegative atom. o Increased dipolarity increases the less electronegative (positively charged) atom to be more susceptible to nucleophilic attack ▪ Anhydrides have two electron withdrawing groups while the smaller amides have nitrogen which is less electronegative than oxygen which means that the dipole is weaker. Conjugation: presence of alternating single and multiple bonds o Implies that all bonds are either sp2 or sp-hybridized, which subsequently implies that there are unhybridized p-orbitals. o If p-orbitals align, resonance can help in delocalizing the pi bond electrons ▪ This would form clouds of electron density above and below the plane of the molecule o Carbonyl-containg compounds can have conjugation established with carbonyl group itself (i.e. – enones) o Makes for very stable structures since the compounds have multiple resonance structures ▪ Allows for the stability of a positive charge once a nucleophile has bonded, which makes them more susceptible to nucleophilic attack Stain in Cyclic Derivatives • • • Certain lactams and lactones are more reactive to hydrolysis because they contain more strain Increased strain reduces resonance which makes hydrolysis more likely. Used in a lot of antibiotics. (use beta-lactams) Nucleophilic Acyl Substitution Anhydride Cleavage • • • Nucleophilic acyl substitution involves nucleophilic attack of the carbonyl carbon with the displacement of the leaving group. All derivatives can participate in these reactions at different rates o Anhydrides are the most reactive, followed by esters and then amides. Cleavage Reaction: splits a molecule into two o Amides can be formed using a cleavage reaction of any carboxylic acid or derivative and ammonia Ammonia acts as nucleophile and carbonyl carbons act as electrophile. Carboxylic acid is the leaving group. Alcohols can also act as nucleophiles towards anhydrides which would result in the formation of esters and carboxylic acids. Anhydrides can be reverted to carboxylic acid by exposing them to water. Anhydride should be symmetric in order to avoid forming a mixture of products. ▪ • • Transesterification • Alcohols act as nucleophiles and displace the esterifying group on an ester. One ester is transformed into another Hydrolysis of Amides • • • Amides can by hydrolyzed under highly acidic conditions via nucleophilic substitution Acidic conditions allow oxygen to become deprotonated which makes it more susceptible to nucleophilic attack. Results in a carboxylic acid and ammonia.