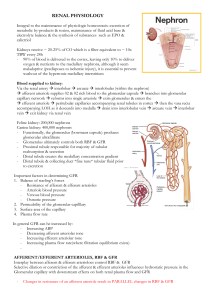
RENAL PHYSIOLOGY Integral to the maintenance of physiologic homeostasis: excretion of metabolic by-products & toxins, maintenance of fluid acid base & electrolyte balance & the synthesis of substances such as EPO & calictriol Kidneys receive ~ 20-25% of CO which is a filter equivalent to ~ 10x TBW every 24h - 90% of blood is delivered to the cortex, leaving only 10% to deliver oxygen & nutrients to the medullary nephrons, although it seem maladaptive (predisposes to ischemic injury), it is essential to prevent washout of the hypertonic medullary interstitium Blood supplied to kidney: Via the renal artery interlobar arcuate interlobular (within the nephron) afferent arteriole supplies 02 & 02 rich blood to the glomerular capsule branches into glomerular capillary network reforms into single arteriole exits glomerulus & enters the efferent arteriole peritubular capillaries accompanying renal tubules in cortex then the vasa recta accompanying LOH as it descends into medulla drain into interlobular vein arcuate vein interlobar vein exit kidney via renal vein Feline kidney: 200,000 nephrons Canine kidney: 400,000 nephrons - Functionally, the glomerulus (bowmans capsule) produces glomerular ultrafiltrate - Glomerulus ultimately controls both RBF & GFR - Proximal tubule responsible for majority of tubular reabsorption & secretion - Distal tubule creates the medullary concentration gradient - Distal tubule & collecting duct *fine tune* tubular fluid prior to excretion Important factors in determining GFR 1. Balance of starling’s forces - Resistance of afferent & efferent arterioles - Arteriole blood pressure - Venous blood pressure - Osmotic pressure 2. Permeability of the glomerular capilllary 3. Surface area of the capillary 4. Plasma flow rate In general GFR can he increased by: - Increasing ABP - Decreasing afferent arteriolar tone - Increasing efferent arteriolar tone - Increasing plasma flow rate(where filtration equilibrium exists) AFFERENT/EFFERENT ARTERIOLES, RBF & GFR Interplay between afferent & efferent arterioloes control RBF & GFR Selective dilation or constriction of the afferent & efferent arterioles influences hydrostatic pressure in the Glomerular capillary with downstream effects on both renal plasma flow and GFR - Changes in resistance of an afferent arteriole result in PARALLEL changes in RBF & GFR - Changes in resistance of an efferent arteriole result in DIVERGENT changes in RBF & GRF Examples: see end of document Ultimately if RBF is insufficient, GFR will be decreased reegardless of the gradient between afferent & efferent arteriolar constriction REGULATION OF RBF, FLUID BALANCE AND ELECTROLYTES 2 important autoregulatory mechanisms ensure that the glomerulus & renal parencyma are not damaged from over or under perfusion 1. Myogenic response a. Stretch is detected by epithelial cells of the afferent arteriole triggering calcium influx & resultant contraction of vascular smooth muscle 2. Tubuloglomerular feedback a. Increased glomerular filtration leads to increased Na & chloride delivery to thick aLOH resulting in release of paracrine agents (ie thromboxane & adenosine) that promote vasocontriction of the afferent arteriole – Tubuloglomerular feedback is dependant on Na/K/2Cl transporter (*blocking transporter with Frusemide impairs this protective response) Both mechanisms autoregulate RBF (& thus GFR) within a MAP 80-170mmHg. Importantly once MAP falls below 80mmHg, the kidney is hypoperfused 4 key systems that regulate RBF: 1. RAAS 2. SNS 3. ADH 4. ANP THE RENIN ANGIOTENSIN ALDOSTERONE SYSTEM Key players Pro-renin: formed in the JGA and cleaved into renin in response to 1-adrenergic stimulation, decreased renal perfusion and reduced sodium reabsorption from renal tubules. Renin: Converts angiotensinogen to angiotensin (Ang) I. Angiotensin converting enzyme (ACE): Converts Ang I to Ang II and Ang III. Conversion of Ang I to Ang II may also occur via the actions of lots of other compounds (details not required for memberships) Ang III has similar actions to Ang II but is less potent. Inactivates bradykinin (potent vasodilator). Inhibits renin release in a negative feedback mechanism. Therefore ACE inhibitors Decrease Na and water reabsorption Decrease aldosterone ECF vol, BP vasodilation systemic arterioles Angiotensin II: • Potent vasoconstrictor acting via the inositol triphosphate pathway in the peripheral endothelial cells to promote calcium release from the sarcoplasmic reticulum (SR). Promotes nADR release in a positive feedback loop, amplifying the vasoconstrictive response. Promotes sodium and water retention via direct effects on the renal tubules and indirectly by stimulating aldosterone production and release. Aldosterone Released from the adrenal gland in response to Ang II stimulation, increased plasma K+ levels, ACTH, catecholamines, endothelin-1 and arginine vasopressin. Circulates to the kidneys, diffuses into the cytoplasm of distal collecting duct (DCT) cells and binds to cytoplasmic mineralocorticoid receptors sodium absorption in the thick ascending loop (TAL), distal tubule (DT) and collecting duct (CD); potassium excretion in the DT and CD. Atrial natriuretic peptide (ANP): secreted by cardiac atria in response to increased stretch (ie increased blood volume or pressure). Decreases BP by decreasing total peripheral resistance (TPR) Increases urinary NaCl and water excretion: Vasodilation of afferent arteriole and vasoconstriction of efferent arteriole ↑ GFR ↓ renin & therefore Ang II secretion ↓ aldosterone–via ↓reninanddirectlyatadrenalcortex ↓ NaCl reabsorption in medullary CD via ↓ aldosterone and directly ↓ ADH secretion and effect ADH/Vasopressin/AVP Vasopressin receptors 1. *V1 receptor: vasoconstriction, platelet aggregation 2. V2 receptor: in the renal DT and CD insertion of water channels in apical membrane ↑ permeability of CD to water increased urine concentration 3. *V3 receptor: potentiation of cortisol release via ACTH * at high levels Other factors influencing ADH release: Increased release: nausea, Ang II, nicotine Decreased release: ANP, ethanol Urodilatin: very similar to but more potent than ANP. Secreted by DT and CD (local action only) in response to increased BP and ECF volume. Results in increased urinary NaCl and water excretion via inhibition of NaCl reabsorption in the medullary CD. Dopamine: released by dopaminergic nerves in the kidneys and possibly synthesised in PT in response to increased ECF vol inhibition of NaCl and water reabsorption in the PT GLOMERULAR FILTRATION Glomerular capillary wall – fenestrations in the capillary endotherlium & filtration slits in visceral epithelium allow small molecules to pass freely into bownas space to form an ultrafiltrate. Ultrafiltrate has a similar composition to plasma Larger negatively charges proteins (ie albumin & Hgb) are restricted from passage across the glomerulus Ultimately, filtration across the glomerular capillaries is reliant on the net effect of Starlins forces - As blood moved through the GC there is net OUTWARD mvmt of fluid, however as oncotic pressure increases along the length of the capillary, filtration DECREASES - An increase in RBF allows the entire SA of the GC to be used for filtration o Leads to an increased filtration co-effeicient & moderate increase in GFR - A decrease in RBF reulsts in a much more substantial decrease in GFR Measurement of GFR 1. Creatinine: freely filtered by the glomerulus - GFR must decline substantially (~75%) before appreciable rise in plasma creatinine concentration occurs Note: urea is not a reliable marker of GFR as it is actively reabsorbed by the renal tubules particularly during low flow states 2. Iohexol clearance test RENAL REGULATION OF EFFECTIVE CIRCULATING BLOOD VOLUME Kidneys adjust Na excretion in response to perceived changes in ECBV and tissue perfusion. Water generally follows sodium... the majority of renal regulation of water is really renal regulation of sodium. ECBV is the portion of the ECF that is in the vascular system and effectively perfusing the tissues. It is affected by the volume and pressure within the blood vessels and CO. In a normal animal, ECBV varies with the volume of ECF, blood pressure and CO. Volume sensors Kidneys Low pressure: Baroreceptors within the walls of the atria and the pulmonary blood vessels. Detect stretch. Decreased filling and stretch stimulates increased sympathetic activity and ADH secretion. Increased stretch of the atria atrial natriuretic peptide (ANP) High pressure: aortic arch, carotid body and the renal afferent arteriole. Respond to blood pressure. Decreased BP increased sympathetic activity and ADH secretion. Juxtaglomerular apparatus (JGA): decreased perfusion of the afferent arteriole increased renin secretion. Increased ECBV: - Decreased renal sympathetic tone - ANP release Together these result in: ↑ GFR: ↑ hydrostatic pressure ↓ Na reabsorption in PT ↓ Na reabsorption in collecting duct (CD): increased delivery of Na to CD overwhelms its ability to reabsorb Na. Resulting in an overall increase in loss of sodium and water Decreased ECBV: 1. ADH release 2. Increased renal sympathetic tone 3. Activation of the RAAS: Resulting in an overall increase in retention of sodium and water RENAL REGULATION OF SODIUM The reabsorption of sodium is a two-step process: 1. Passive movement Na from the tubular fluid into the tubule cell. 2. Active extrusion of Na from the tubule cell into the blood. This process requires energy at utilizes the all-important Na-K-ATPase pump. Cotransporter: a membrane protein involved in the transport of two (or more) different molecules or ions across the membrane at the same time. May be a: - Symporter –molecules moved in the same direction - Antiporter – molecules moved in the opposite directions. To maintain electroneutrality, when sodium is moved across a membrane, it is either accompanied by a negative ve ion, or exchanges for another positive ion. Metabolic alkalosis caused by the loss of upper GI contents (eg gastric obstruction in the dog leading to vomiting), leads to a situation of both hypovolaemia and hypochloraemia. The kidneys priorities are: - Volume regulation - Acid-base regulation To reabsorb Na in metabolic alkalosis, Na is reabsorbed with a negative ion. The animal is hypochloraemic (HCl loss in vomit) so the kidney resorbs Na with HCO3-.This allows Na reabsorption and helps to retain water in the face of hypovolaemia, BUT perpetuates the metabolic alkalosis. The situation is made worse by decreased ECBV stimulating the release of aldosterone: Na is exchanged for H+ and K+ in the DT worsening the alkalosis and hypokalaemia Treatment involves providing fluid that is higher in Cl than we might otherwise use to restore adequate circulating volume, and replace the chloride deficit. As well as removing the cause of the obstruction. RENAL REGULATION OF POTASSIUM The large concentration difference between intracellular and extracellular K is maintained by the Na-K-ATPase pump. Factors affecting K distribution: - Insulin intracellular movement - Aldosterone K uptake into cells and urinary excretion - Adrenalin activation of α (release of K from cells) and β2 (K uptake by cells) receptors Acid-base balance: Metabolic acidosis, especially inorganic acids decreases renal potassium secretion Metabolic alkalosis ↓ K: K moves in to cells in exchange for H+ - Osmolality: For every 10mOsm increase in serum osmolality, potassium increases o by 0.4 – 0.8 mmol/L. This is secondary to water loss from cells resulting in increase o concentration of K in cells leading to K efflux - Cell Lysis - Exercise o Potassium is freely filtered with glomerular fluid. Potassium loss is proportional to fluid flow along the distal tubule, ie urine output, therefore diuretics increase K excretion. - When potassium levels are low: Reabs filtered K occurs 67% PT, 20% TAL, 3% DT, 9% CD; 1% filtered K excreted. - When K levels are normal or increased: K is still reabs in PT and TAL but secreted by DT and CD. Up to 80% filtered K excreted, mainly due to aldosterone: ↑ K > 24 hrs increased number and function of Na-K-ATPase pumps in principal cells. Aldosterone also increases K uptake into cells. RENAL REGULATION OF CALCIUM Parathyroid hormone: minute – minute control of Ca++: ↑ osteoclast numbers and function. Calcitriol: day – day control: differentiation of osteoclasts. Vit D (calciferol) Dietary vitamin D is hydroxylated in the liver to calcidiol Calcidol is hydroxylated in the kidney by 1α hydroxylase to calcitriol, the active metabolite. Calcitriol: - Intestine: ↑ Ca++ and P abs from gut Bone: necessary for bone formation and mineralisation Kidney: direct –ve feedback by inhibiting 1α hydroxylase ↑Ca++ and P reabs from filtrate Prevents excessive PTH production in CRF, renal protection too Parathyroid gland: direct inhibition of PTH synthesis Indirect inhibition of PTH via ↑Ca++ abs in intestine Suppression of PTH synthesis cannot occur without calcitriol even in o hypercalcaemia. Parathyroid Hormone: ↑ Ca++ via bone and kidney Fine control of Ca++ levels: large changes in levels of PTH (400%) with small changes (2%) in Ca++ levels RENAL SECONDARY HYPERPARATHYROIDISM: Renal failure ↓ calcitriol production decreased intestinal Ca++ abs ↓ sensitivity of parathyroid receptors to iCa++ and calcitriol increased PTH ↓ GFR ↑PO4 ↓ Ca++ (mass law effect) Metabolic acidosis ↑ proportion of calcium is ionised Ca++ ↑PO4 and ↓Ca ↑ PTH and bone reabs RENAL REGULATION OF ACID BASE BALANCE The concentration of H+ in the body is very low compared with other ions. Metabolism generates acids. CO2 is a volatile acid and is eliminated via ventilation. Non- volatile acids such as sulphuric acid, phosphoric acid, and other organic acids and non- volatile bases (for our purposes, bicarbonate) that cannot be eliminated by the lungs. The kidneys are then responsible for regulating hydrogen ion excretion and regenerating bicarbonate ions to maintain extracellular fluid pH within very narrow limits. The kidney is the only regulated route of H+ loss. Renal regulation: Reabs of almost all filtered HCO3 Regeneration of HCO3Proximal tubule: H excretion to facilitate HCO3 reabsorption It is all about Na! Na:H antiporter Up to 80% filtered HCO3- reabs in PT. HCO3- - Na cotransport at basolateral membrane. The maximal filtered load changes with available Na: - Hypovolaemia leads to ↑ Na reabsorption via RAAS: Na-H (and Na-K) exchange across apical membrane in the PT H is excreted and HCO3 is reabs alkalosis and hypokalaemia perpetuated to retain volume o If there is enough Cl in the filtrate, Na and Cl are reabsorbed together with out excessive reabsorption of HCO3- and alkalosis does not develop o If there is decreased Cl, HCO3- is reabsorbed with Na and alkalosis develops; also get ↑H+ - excretion to exchange for Na therefore paradoxical aciduria. o If there is concurrent hypokalaemia, intracellular shift of H, worsens alkalaemiA Fluid overload leads to ↓ Na reabs and therefore ↓ HCO3- reabs Collecting Duct: H+ excretion to get rid of acid; resorption and secretion of HCO3 by intercalated cells RENAL RESPONSE TO METABOLIC ACIDOSIS: ammonium excretion ↑ NH4 excretion leads to Cl loss and regeneration of HCO3 (except cats probably). Response in 3 – 5 days. Ammonium is produced from glutamate in the PT, H+ combines with NH3 in tubule cells to form NH4 (Ammonium) Acidosis: NH4+ excreted by kidneys and 2 “free” HCO3 regenerated Alkalosis/normal: NH4+ used by liver to make urea, 2 HCO3consumed no net change RENAL RESPONSE TO METABOLIC ALKALOSIS: decreased HCO3- reabs The kidney is better at excreting an alkaline load than an acid load as long as there is adequate Na and ECBV. Renal HCO3- excretion isimpaired if there is ↓ GFR, ↓ K, ↓ Cl, ↓ ECBV or a stimulus to retain Na in the face of a (relative) Cl deficit (the vomiting dog example above) ↑ CO2 ↑ plasma HCO3- by a direct ↓ in renal HCO3- excretion. RENAL REGULATION OF GLUCOSE Glucose is filtered & reabsorbed in the PT by Na dependant carrier transport mechanisms Renal threshold for glucose: Dog: 180-200mg/dl (10-11.1mmol/l) Cat: 280-290mg/dl (15.5-16.1mmol/l) Note: An adequately concentrated urine indicated the renal tubules can dilute and concentrate urine making renal disease unlikely (but doesn’t r/o) An inadequately concentrated urine in the face of dehydration indicates impairment of the ability of the tubules to concentrate urine or create a hypertonic medullary interstition or respond to ADH (without tubular disease)