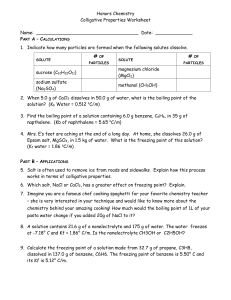
Lesson 4 Colligative Properties of Solutions Focus Question Why do we salt the roads when it's cold outside? New Vocabulary colligative property vapor pressure lowering boiling point elevation freezing point depression osmosis osmotic pressure Review Vocabulary ion: an atom that is electrically charged Colligative Properties • Colligative properties are physical properties of solutions that are affected by the number of particles but not by the identity of dissolved solute particles. • Colligative means depending on the collection. • Colligative properties include: • Vapor pressure lowering • Boiling point elevation • Freezing point depression • Osmotic pressure Electrolytes • Ionic compounds are electrolytes because they dissociate in water to form a solution that conducts electricity. • Electrolytes that produce many ions are strong electrolytes. • Electrolytes that produce only a few ions are weak electrolytes. • Many molecular compounds do not ionize when dissolved and do not conduct electricity. These are called nonelectrolytes. • There are some exceptions; molecular compounds that do ionize are electrolytes. Vapor Pressure Lowering • Recall that vapor pressure is the pressure exerted in a closed container by the particles that have escaped the liquid’s surface and entered the gaseous state. • Adding a nonvolatile solute (one that has little tendency to become a gas) to a solvent lowers the solvent’s vapor pressure. • Vapor pressure lowering depends on the number of solute particles in solution. It is a colligative property of solutions. Vapor Pressure Lowering • When a solute is present, a mixture of solvent and solute occupies the surface area, and fewer particles enter the gaseous state. • The greater the number of solute particles, the lower the vapor pressure. Boiling Point Elevation • When a nonvolatile solute lowers the vapor pressure of a solvent, the boiling point is also affected. • More heat is needed to supply additional kinetic energy to raise the vapor pressure to atmospheric pressure. Boiling Point Elevation • The temperature difference between a solution’s boiling point and a pure solvent's boiling point is called the boiling point elevation, ΔTb. • For non-electrolytes, the boiling point elevation is directly proportional to the solution’s molality. Boiling Point Elevation Freezing Point Depression • At a solvent's freezing point temperature, particles no longer have sufficient kinetic energy to overcome interparticle attractive forces. • The freezing point of a solution is always lower than that of the pure solvent. • Solute particles interfere with the attractive forces among solvent particles. Freezing Point Depression • A solution's freezing point depression, ΔTf, is the difference in temperature between its freezing point and the freezing point of the pure solvent. • Like boiling point elevation, freezing point depression is a colligative property of a solution and is directly proportional to molality. Freezing Point Depression CHANGES IN BOILING AND FREEZING POINTS Use with Example Problem 6. Problem Sodium chloride (NaCl) is often used to prevent icy roads and to freeze ice cream. What are the boiling and freezing points of a 0.029m aqueous solution of sodium chloride? Response ANALYZE THE PROBLEM You are given the molality of an aqueous sodium chloride solution. First, calculate ΔTb and ΔTf based on the number of particles in the solution. Then, to determine the elevated boiling point and the depressed freezing point, add ΔTb to the normal boiling point and subtract ΔTf from the normal freezing point of water. KNOWN UNKNOWN molality of solution = 0.029m boiling point = ?°C Kb = 0.512°C/m freezing point = ?°C Kf = 1.86°C/m SOLVE FOR THE UNKNOWN Determine the molality of the particles. Particle molality = 2 × 0.029m = 0.058m Determine ΔTb and ΔTf • State the boiling point elevation and freezing point depression formulas. ΔTb = Kbm ΔTf = Kfm CHANGES IN BOILING AND FREEZING POINTS SOLVE FOR THE UNKNOWN (continued) • Substitute Kb = 0.512°C/m, Kf = 1.86°C/m, and m = 0.058m. ΔTb = (0.512°C/m)(0.058m) = 0.030°C ΔTf = (1.86°C/m)(0.058m) = 0.11°C Determine the elevated boiling point and depressed freezing point of the solution. • Add ΔTb to the normal boiling point and subtract ΔTf from the normal freezing point. boiling point = 100.000°C + 0.030°C = 100.030°C freezing point = 0.00°C - 0.11°C = -0.11°C EVALUATE THE ANSWER The boiling point is higher and the freezing point is lower, as expected. Because the molality of the solution has two significant figures, both ΔTb and ΔTf have two significant figures. Because the normal boiling point and freezing point are exact values, they do not affect the number of significant figures in the final answer. Changes in Boiling and Freezing Points Osmotic Pressure Osmosis is the diffusion of a solvent through a semipermeable membrane. Osmotic Pressure • Osmotic pressure is the amount of additional pressure caused by water molecules that move into the concentrated solution. • Osmotic pressure depends on the number of solute particles in a given volume of solution and is a colligative property of solutions. Quiz 1. When a nonvolatile solute is added to a solvent, vapor pressure ______. A decreases B increases C stays the same D disappears CORRECT Quiz 2. For non-electrolytes, boiling point elevation and freezing point depression are both: A directly proportional to the solution’s molarity B inversely proportional to the solution’s molarity C directly proportional to the solution's molality CORRECT D inversely proportional to the solution's molality Quiz 3. The freezing point of a solution is always ______ the freezing point of the pure solvent. A higher than B lower than C the same as D unrelated to CORRECT Quiz 4. What process does this figure illustrate? A vapor pressure lowering B boiling point elevation C freezing point depression D osmosis CORRECT