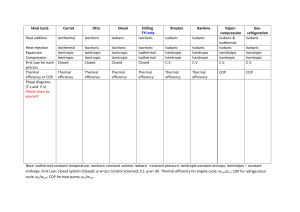
Ng, Matt Vincent T. SCIENVP A60 Thermodynamics Thermodynamics is the branch of physics that deals with heat and other energy. It explains how thermal energy were converted to energy. Thermodynamics measures the energy which can be complicated because it consists of large numbers of molecules or atoms. Thermodynamics have several properties one of them is heat. Heat is energy that is transferred from one body to another meaning to say it is being transferred because of temperature difference. Heat is also conserved. It cannot be created nor destroyed. Heat can also be converted to other form of energy. Another property is temperature; temperature is the amount of heat transferred to the substance. It is also the measurement of hotness and coldness. Temperature measures the average kinetic energy of the particles in a substance. Temperature has its own scale and it is called Celsius which is based from freezing and boiling point of water, they also their own assigned values of 0-degree C and 100-degree C. Fahrenheit is also used in temperature and it is the same as Celsius but different values. For Fahrenheit, the values are 32 F and 212 F. Kelvin is also used in temperature and it is the same as Celsius and Fahrenheit. The only difference is that it starts from absolutely zero. There are other properties like Pressure, the normal component of force. Volume, the quantity of space possessed by a material. Entropy, the quantity of disorder possessed by material and internal energy, the energy of a material which is due to the kinetic and potential energy. There are also two properties in thermodynamic, the intensive, which is the independent of the quantity of materials, and the extensive, which are directly proportional to the quantity of material. Pseudointensive properties are extensive properties that expressed the unit quantity of materials and under that there are the specific properties, expressed in a unit mass basis, and the molar properties, expressed in a mole basis. Thermodynamic didn’t only have properties but it also has laws that needed to be followed. One law is the “Zeroth Law”. It is stated that if two bodies are in thermal equilibrium with some third body. Then they are also equilibrium with each other. The second law is the “First Law”. It is stated that the total increase in energy of a system is equal to the increase of thermal energy plus the work done in the system. The third law is the “Second Law”. It is stated that it cannot be transferred from a body at a lower temperature to a body with higher temperature without an additional energy. The last law is the “Third Law”. It is stated that the entropy of a pure crystal should be zero. Because entropy is a waste energy. It is an energy that can no longer work. Thermodynamics also have process and cycle. Process is one, it is any succession of events. Chemical process, is a chemical or physical operation or series. Thermodynamic process, it is a path of succession of states through which the system passes in moving from the intial to the final state. Polytropic process, it is usually associated only to the system for which the ideal gas assumptions hold. Under Polytropic, there are four processes, 1. Isobaric (constant pressure)2. Isothermal (constant temperature) 3. Isentropic (constant entropy) 4. Isochoric (constant volume). Two other important process are the adiabetic, no heat is being transferred and the isenthalpic, constant enthalpy. Four common idealized thermodynamic cycle are the Carnot, Rakine, Otto, and Diesel Cycle. Carnot cycle is isothermal and isentropic compressed and they followed by isothermal and isentropic expansion. Rankine cycle is isobaric and isentropic compressed and followed by isobaric and isentropic expansion. Otto cycle is isentropic and isochoric compressed and followed by isentropic and isochoric expansion. And Diesel cycle is isentropic compressed and followed by isobaric, isentropic and isochoric expansion. Reference: http://sunset.backbone.olemiss.edu/~cmpcs/engr321def.html https://www.livescience.com/50776-thermodynamics.html