Thermodynamics
advertisement
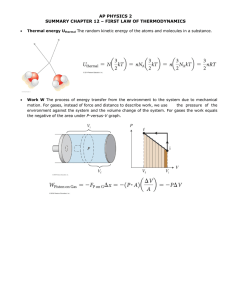
Thermodynamics Thermodynamics • The study of processes in which energy is t transferred f d as heat h t and d as work. k • Thermodynamics system – Object or set of objects we wish to consider (everything else is environment) – Could be closed or open system 1 1st Law of Thermodynamics • The change in internal energy of a closed system t will ill b be equall tto th the h heatt added dd d tto the system minus the work done by the system ΔU = ΔQ − ΔW internal energy work heat ΔQ = ΔU + ΔW 1st Law • This law is really just a statement of conservation ti off energy. • Q and W represent energy transferred into and out of the system. 2 Notes: • • • • Work done on the system is negative Work done by the system is positive Heat added to the system is positive Heat lost by the system is negative Thermodynamic Processes 3 Isothermal Process • Temperature is constant t t • Internal energy does not change Q = ΔU + W ΔU = 0 Q =W 4 Adiabatic Process • No heat is transferred • Well insulated container, quick compression or expansion Q = ΔU + W Q=0 ΔU = −W 5 Isobaric Process • Pressure is constant • Work is done: W = Fd P= F A W = PAd W = PΔV 6 Isochoric (Isovolumetric) • No N work k iis d done • Volume is constant 7 Work Done by a Gas • Consider the following thermodynamic cycle 8 Work done by a gas as it expands Work done on a gas as it is compressed 9 Net work done by the gas 10