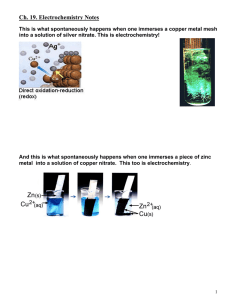
Ch. 19. Electrochemistry Notes This is what spontaneously happens when one immerses a copper metal mesh into a solution of silver nitrate. This is electrochemistry! And this is what spontaneously happens when one immerses a piece of zinc metal into a solution of copper nitrate. This too is electrochemistry. 1 19.2 Electrochemical Cells Voltaic (Galvanic) Cells (batteries), are spontaneous and consist of two ½-cells. Each ½-reaction takes place in a separate ½-cell. Combining the two ½-cells makes the full cell. Constructing a complete cell from two half-cells requires indirect connections between electrodes: A salt bridge and a device connector. The Cu-Zn Voltaic Cell: electrons flow spontaneously from anode to cathode 2 Cell Diagrams are short-hand notations for the electrochemical cell. Only the chemical reactions are explicitly written. Physical separations in the cell are represented by vertical lines. Examples. Cu-Zn cel,l above. Fe-MnO4 cell, below. ││ ││ 3 19.3 Standard Potentials This Table lists half reaction according to a property called the Standard Reduction Potential, E°red, (Gilbert, A-6). These are also called standard half-cell reduction potentials. 4 Construction of such a table requires a reference point: The Standard Hydrogen Electrode. 19.5 The Standard Hydrogen Electrode: SHE 2H+(aq) + 2e- = H2 (g) E°red = E1/2° = 0.000V One can measure any ½-Cell against the SHE to establish a table of electrode potentials. Interpretation of Standard Reduction Potentials. Each electrode is assigned a standard reduction potential, E°red; also called a standard half-cell potential (written as a reduction rexn), E1/2°, also called a standard electrode potential. In a voltaic cell, two half-cells are connected. (Like the cell on the next page). Each ½-cell has its own charge and its own electrode potential. When the two ½-cells are connected electrons flow spontaneously from the higher potential energy electrode (the one with greater negative charge) to the lower potential energy electrode (the one with less negative or more positive charge). Elect. Electrode P.E. Charge + Rationalize this with the observation that in the cell shown on the next page Zn(s) is spontaneously oxidized to Zn2+(aq). 5 The relationship between half-cell potentials and the Cell Potential. E°cell = E°cathode - E°anode In the case of cell constructed from the SHE and a Cu/Cu2+ electrode copper is observed not to be oxidized. Draw and analyze this cell. 6 Using Tables of Standard Reduction Potentials. Predicting the spontaneous direction of a redox reaction. E°cell = E°cathode - E°anode = POSITIVE VALUE Prediction of spontaneity and Voltaic Cell components E°cell, ΔG° & Keq A. ΔG° = - n F E°cell B. R T ln Keq = ΔG° = - n F E°cell => 7 19.6 Cell Potential also depends on the concentrations of the ½-cell constituents. (Non-Standard Cell Potentials). The Nernst Equation describes how the cell potential at non-standard conditions (Ecell) relates to the standard cell potential (E°cell). 8 Concentration cells are the basis for useful things, like pH meters and ion-specific electrodes. 9 An Actual pH combination electrode. Friday April 19, actual lecture notes: The Lead Storage Battery. Most cars have them. 10 11 21st century energy. Hydrogen-Oxygen Fuel Cell. No Pollution. 12 Where with the Hydrogen and Nitrogen Come From? The Seas” 13 Electolytic cells reverse the current flow by using an External Power Source, which in this case is a battery. Reverses the half-cell reactions in each compartment. End Electrochemistry Notes. 14