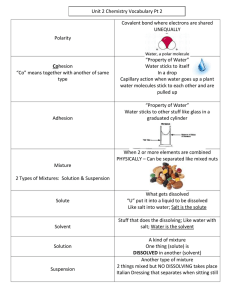
CLASSIFICATION OF MATTER & PROPERTIES MATTER ●Matter: everything that occupies a space in the universe ●Every kind of matter is made of one or more elements • What is an atom? Atoms are the smallest particle of matter, and of an element. • What is an element? An element is a substance consisting of atoms which all have the same number of protons. • What is a molecule? Two or more atoms chemically combined. How can matter be classified? • Matter is made up of basic “ingredients” known as atoms. • An atom is the smallest unit of an element that maintains the properties of that element. • Substances are classified as elements, compounds, and mixtures. Basic ingredients of all “stuff” Substances are classified as: Matter Elements Compounds Mixtures How can matter be classified? • An atom is a building block of matter • An element is made of only one kind of atom. • A compound is made up of different kinds of atoms that are chemically combined. • A mixture contains a variety of elements and compounds that are not chemically combined. Classification of Matter https://chemistrygod.com/classification-of-matter Pure Substances ● Always have the same composition and are formed by chemical processes. ● Either elements or compounds. ● Examples: ▪ Pure water (H2O), carbon dioxide (CO2), hydrogen (H2), gold (Au) Copyright © Cengage Learning. All rights reserved Element ● A substance that cannot be broken down into other substances by chemical methods. ● Examples: ▪ Iron (Fe), aluminum (Al), oxygen (O2), and hydrogen (H2) ● All of the matter in the world around us contains elements. Elements • Elements are classified as metals, nonmetals, or metalloids. • Metals: shiny, conduct electricity, malleable, ductile • Nonmetals: not shiny, do not conduct electricity or heat. • Metalloids: have properties of both • The periodic table is a tool used to classify and identify elements that have similar properties. Compound ● A substance composed of a given combination of elements that can be broken down into those elements by chemical methods. ● Examples: ▪ Water (H2O), carbon dioxide (CO2), table sugar (C12H22O11) ● A compound contains atoms of different elements. ● A compound always has the same composition (same combination of atoms). Copyright © Cengage Learning. All rights reserved Compounds • Compounds can be classified as acidic, basic, or neutral by measuring pH. • Pure water (Neutral) has a pH of 7 • Acids have a pH below 7 • Bases have a pH above 7 Compounds • Compounds can also be organic or inorganic based on their composition. • Organic compounds are those that contain only carbon, oxygen and hydrogen. • Organic compounds made by living things are called biochemical molecules or bio-molecules. • Carbohydrates, lipids, proteins, and nucleic acids are biomolecules Mixtures • A mixture is a combination of two or more substances that are combined physically but not chemically. • Mixtures are not pure substances and do not have definite properties. • Substances within a mixture keep their identities and individual properties. • Mixtures can be separated by physical changes, although some mixtures are difficult to separate. Mixtures ● Have variable composition. ● Examples ▪ Wood, wine, coffee, granite ● Can be separated into pure substances: elements and/or compounds using physical processes. Homogeneous Mixture ● Same throughout. ● Having visibly indistinguishable parts. ● Does not vary in composition from one region to another. Apple Juice Cranberry Juice Orange Juice with ice Homogeneous Mixture – Examples ● Air ● Brass ● Table salt stirred into water ● Coffee Heterogeneous Mixture ● Having visibly distinguishable parts. ● Contains regions that have different properties from those of other regions. Heterogeneous Mixture – Examples ● Oil and vinegar dressing ● Sand ● Soup Copyright © Cengage Learning. All rights reserved ● Mixtures can be separated based on different physical ● Magnets, centrifuges, filters, and other materials can be used to separate mixtures. properties of the components. Different Physical Property Technique Boiling point Distillation State of matter (solid/liquid/gas) Adherence to a surface Chromatography Volatility Evaporation Filtration Copyright © Cengage Learning. All rights reserved Distillation of a Solution Consisting of Salt Dissolved in Water Copyright © Cengage Learning. All rights reserved ● No chemical change occurs when salt water is distilled. Copyright © Cengage Learning. All rights reserved Solution - Homogeneous Mixture ●A physical mixture of two or more substances ●Composed of solutes and solvents the substance in the larger amount that dissolves the solute the substance in the smallest amount and the one that dissolves in the solvent Iced Tea Mix (solute) Salt water is considered a solution. How can it be physically separated? Iced Tea (solution) Water (solvent) ○ A solution is a homogeneous mixture of ions or molecules of two or more substances. ○ Two parts ■ Solvent is the component that is in the largest quantity ■ Solute is the component that is dissolved in the solvent. ○ If one of the components of a solution is a liquid it is usually the solvent. ○ If the solvent is water then the solution is identified as an aqueous solution. Solutes Change Solvents ●The amount of solute in a solution determines how much the physical properties of the solvent are changed ●Examples: Lowering the Freezing Point The freezing point of a liquid solvent decreases when a solute is dissolved in it. Ex. Pure water freezes at (00C), but when salt is dissolved in it, the freezing point is lowered. This is why people use salt to melt ice. Raising the Boiling Point The boiling point of a solution is higher than the boiling point of the solvent. Therefore, a solution can remain a liquid at a higher temperature than its pure solvent. Ex. The boiling point of pure water is (1000C), but when salt is dissolved in it, the boiling point is higher. This is why it takes salt water longer to boil than fresh water. Concentration ● The amount of solute dissolved in a solvent at a given temperature • described as dilute if it has a low concentration of solute. • described as concentrated if it has a high concentration of solute. When the solution reaches the maximum concentration of solute that can be diluted by the solute it is described as saturated. • described as supersaturated if contains more solute than the amout the solute can disolve. INFORMATION THAT SHOULD BE INCLUDED IN YOUR NOTEBOOKS 1. Definition of matter, atom, element and molecule 2. What makes one element different from other element? 3. A graphic organizer with the classification of matter 4. Define element, pure substance, compound and give 4 examples of each one. 5. Define homogeneous mixture and give 4 examples 6. Define heterogeneous mixture and give 4 examples 7. A graphic organizer with the classification of solutions that includes 2 examples or each one. HOMEWORK We are working on a lab that will be developed on your kitchen, please make sure you have a grown up to help you with the cooking procedure. Each student should bring: ● 2 plastic/ glasses ● 2 cups of sugar ● 1/2 cups of water ● 2 wood sticks ● 4 clothes peg/gripper ● food coloring ● essences or sweet flavors ● 1 old newspaper ● 1 small pot ● 1 plastic or wooden spoon PROPERTIES OF MATTER PROPERTIES OF MATTER Matter has different qualities that characterize it. We can distinguish two types of properties: ●Physical ●Chemical PHYSICAL PROPERTIES The physical properties are those that can be observed with our senses, without changing the structure of matter, and are used to identify, describe and classify matter. For example: ●Mass ●Weight ●Volume ●Porosity, Shape, Texture, Color, Shape ●State Mass ●A measure of how much matter is in an object. Weight ●A measure of the force of gravity on an object. Volume ●The amount of space that matter occupies. Porosity, texture, size, color and shape States of Matter ●There are different “states” of matter. Elements and compounds can move from one phase to another when special physical forces are present. ●Solid ●Liquid ●Gas The striking blue walls in this photo are actually the sheer ice walls of a massive glacier. The glacier in the picture is in Argentina, and the bluish water in the foreground is Lake Argentina. The photo represents an important concept in physical science. Can you guess what it is? ●The photo represents water in three states of matter at the same time. ●There are actually four well-known states of matter: solid, liquid, gas, and plasma. Plasma isn’t represented in the iceberg photo, but the other three states of matter are. The iceberg itself consists of water in the solid state, and the lake consists of water in the liquid state. ●Q: Where is water in the gaseous state in the above photo? ●A: You can’t see the gaseous water, but it’s there. It exists as water vapor in the air. Solids ●A solid has its own shape. ●A solid does not change unless you cut, bend, or break it. ●Solids have a define volume defined shape and have a defined mass. ● Particles are very close together Liquids ●Liquids do not have their own shape. ●Liquids take the shape of their container. ●Liquids have an undefined shape, defined volume and defined mass. ●Particles are not very close Gases ●Gases have no defined size or shape. ●Gases take the shape of its container. ●A gas will fill all the space inside a container. ●Gases have undefined shape, undefined volume and defined mass ● Particles are far away and move freely Review: 1. Define state of matter. 2. Which states of matter are most common on Earth? 3. Make a table comparing and contrasting solids, liquids, and gases. CHEMICAL PROPERTIES The chemical properties are those that tell us how matter change when combined with other substances , for example: ●Conductivity ●Dissolved oxygen ●Hardness ●pH pH What does it mean for a solution to be acidic or basic (alkaline)? An acid is a substance that donates hydrogen ions. A base is a substance that accepts hydrogen ions. Acidity and alkalinity are measured with a logarithmic scale called pH. ● A strongly acidic solution can have one hundred million million (100,000,000,000,000) times more hydrogen ions than a strongly basic solution! The flip side, of course, is that a strongly basic solution can have 100,000,000,000,000 times more hydroxide ions than a strongly acidic solution.