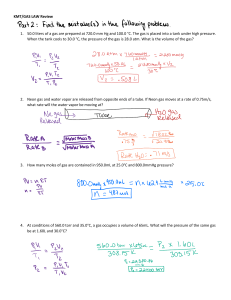
Name___________________________ Block: _____ PS 7.2 - Which Gas Law do I use? Boyle’s law Charles’ Law Gay-Lussac’s Law P1V1 = P2V2 V1 V2 T1 T2 P1 P2 T1 T2 Use one of the above equations to complete the following problems. 1. If 9.0 liters of air are heated from 20.0°C to 120.0°C, what will the new volume be? 2. 400.0mL of helium are under a pressure of 1.0 atm. What pressure, in atmospheres, is necessary to compress the gas to 50.0mL, at constant temperature? 3. A cylinder contains a gas which has a pressure of 125kPa at a temperature of 200 K. Find the temperature of the gas which has a pressure of 100kPa. 4. A gas has a volume of 100.0mL at 36.0°C. What volume will it occupy at standard temperature, 0.0°C, assuming pressure is constant? 5. A weather balloon contains 12.0 liters of hydrogen at 750 mmHg. Assuming constant temperature, at what pressure will the gas occupy a volume of 20.0 liters? 6. A bubble of carbon dioxide gas in some unbaked bread dough has a volume of 1.15mL at a temperature of 22.0°C. What volume will the bubble have when the bread is baked and the bubble reaches a temperature of 99.0°C? 7. A gas cylinder contains 0.722L of hydrogen gas at a pressure of 10.6 atm. If the gas is used to fill up a balloon at a pressure of 0.96atm, what is the volume, in L, of the filled balloon?