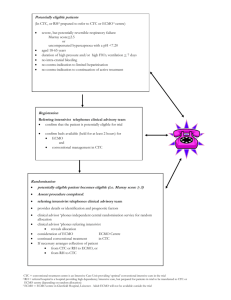
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/314216150 Trends in Extracorporeal membrane oxygenation(ECMO) nursing Presentation · October 2015 DOI: 10.13140/RG.2.2.28102.06728 CITATIONS READS 0 409 1 author: Sonia Cherian Dubai Hospital, Dubai Health Authority, UAE 11 PUBLICATIONS 11 CITATIONS SEE PROFILE Some of the authors of this publication are also working on these related projects: Leadership in nursing View project Improve care of patients on ECMO in Criticare units of Dubai Hospital View project All content following this page was uploaded by Sonia Cherian on 04 March 2017. The user has requested enhancement of the downloaded file. Trends in Extracorporeal membrane oxygenation (ECMO) nursing Sonia Cherian DH Objectives: At the end of the lecture the staffs will be able to : • Understand the meaning of ECMO & how it works • Explain the types of ECMO & how it works • Verbalise the difference between ECMO and CPB • List down the various indications & contraindications for ECMO • Explain the complications of ECMO • Ennumerate the various nursing implications for a patient on ECMO • Verbalise the ECMO emergencies • Understand the use of ECMO in neonates & pediatrics Outline: • • • • • • • • • • • • • Brief history & meaning Difference between ECMO and CPB How does ECMO work Types of ECMO & their differences Indications Contraindications Complications Special considerations Nursing implications ECMO Emergencies Documentation ECMO in Neonates & Pediatrics Outcomes & Future History of ECMO • In May 1953, Gibbon used artificial oxygenation and perfusion support for the first successful open heart operation. • In 1954, Lillehei developed the cross-circulation technique by using slightly anesthetized adult volunteers as live cardiopulmonary bypass apparatuses during the repair of certain congenital cardiac disorders. • In 1965, Rashkind and coworkers were the first to use a bubble oxygenator as support in a neonate dying of respiratory failure. • In 1969, Dorson and colleagues reported the use of a membrane oxygenator for cardiopulmonary bypass in infants. • In 1970, Baffes et al reported the successful use of extracorporeal membrane oxygenation as support in infants with congenital heart defects who were undergoing cardiac surgery. • In 1975, Bartlett et al were the first to successfully use ECMO in neonates with severe respiratory distress. What is ECMO? • ECMO (Extra-Corporeal Membrane Oxygenation) is an extracorporeal technique of providing both cardiac and/or respiratory support oxygen to patients whose heart and/or lungs are severely diseased or damaged and can no longer serve their function. ECLS • The term extracorporeal life support (ECLS) denotes the use of prolonged extracorporeal cardiopulmonary bypass, usually via extrathoracic cannulation, in patients with acute, reversible cardiac or respiratory failure who are unresponsive to conventional medical or pharmacologic management. • ECMO is the traditional term associated with this technique, ECLS is the current, preferred mnemonic since the term "life support" encompasses functions other than "oxygenation", including cardiac and hemodynamic support as well as carbon dioxide elimination. Is it a therapeutic intervention? • It is important to recognize that ECLS is not a therapeutic intervention. • Instead, ECLS simply provides cardiopulmonary support so that the patient is spared the deleterious effects of high airway pressure, high FiO2, and perfusion impairment • During ECLS, "reversible" pathophysiologic processes are allowed to resolve either by spontaneous means or by medical or surgical therapeutic intervention. How is it different from Cardiopulmonary bypass ECMO 1. frequently instituted using only cervical cannulation, which can be performed under local anesthesia 2. used for longer-term support ranging from 310 days 3. Purpose - to allow time for intrinsic recovery of the lungs and heart Cardiopulmonary Bypass 1. usually instituted by transthoracic cannulation under general anesthesia 2. used for short-term support measured in hours 3. Purpose – Temporary support during various types of cardiac surgical procedures. • The roller pump causes less hemolysis, • The venous reservoir is used with the roller pump for neonatal ECMO. • The oxygenator is responsible for exchanging both oxygen and carbon dioxide • The heat exchanger warms the blood using a countercurrent mechanism. Safety Devices/Monitors: • Air bubble detectors can identify microscopic air bubbles in the arterialized blood and automatically turn off the blood pump. • Arterial line filters between the heat exchanger and the arterial cannula are used to trap air, thrombi, and other emboli. • Pressure monitors, which are placed before and after the oxygenator, measure the pressure of the circulating blood and are used to monitor for a dangerous rise in circuit pressure- critical in preventing circuit disruption in the face of distal occlusion. • A continuous venous oxygen saturation monitor and temperature monitor are other important safety features. Types: There are several forms of ECMO, the two most common of which being 1.Veno-arterial (VA) 2.Veno-venous (VV). 3.Double lumen Veno-venous (DLVV) In both modalities, blood drained from the venous system is oxygenated outside of the body. In VV ECMO, no cardiac support is provided. Veno-Arterial ECMO: • A venous cannula - placed in the right common femoral vein for extraction . • An arterial cannula - placed into the right femoral artery for infusion. • The tip of the femoral venous cannula should be maintained near the junction of the inferior vena cava and right atrium, while the tip of the femoral arterial cannula is maintained in the iliac artery. Central VA ECMO: Central VA ECMO - with cannulae in the right atrium and ascending aorta • This method may be used if cardiopulmonary bypass has already been established. • Transthoracic cannulation allows left heart decompression by cannulation of the left atrium. This is useful in patients with primary left heart failure. Veno-Venous (VV): • In Veno-venous ECMO – the blood is returned to the venous system. • Venous cannulae are usually placed in the right common femoral vein for drainage and right internal jugular vein for infusion. • Alternatively, a dual lumen catheter is inserted into the right internal jugular vein, draining blood from the superior and inferior vena cavae and returning it to the right atrium Differences Between Venoarterial and Venovenous Extracorporeal Membrane Oxygenation Venovenous ECMO Venoarterial ECMO Indications: Criteria for the initiation of ECMO include acute severe cardiac or pulmonary failure that is potentially reversible and unresponsive to conventional management Hypoxemic respiratory failure with a ratio of arterial oxygen tension to Fraction of Inspired Oxygen (PaO2/FiO2) of <100 mmHg despite optimization of the ventilator settings, including the FiO2, PEEP, and I:E ratio. Hypercapnic respiratory failure with an arterial pH <7.20 Refractory cardiogenic shock Cardiac arrest Failure to wean from cardiopulmonary bypass after cardiac surgery As a bridge to either cardiac transplantation or placement of a ventricular assist device Contraindications: The relative contraindications are: 1.Conditions incompatible with normal life if the patient recovers 2.Preexisting conditions that affect the quality of life (CNS status, end stage malignancy, risk of systemic bleeding with anticoagulation) 3.Age and size of patient 4.Futility: patients that are too sick, have been on conventional therapy too long, or have a fatal diagnosis. Initiation, Titration & Maintenance Initiation Performed only by expert The patient is anticoagulated with intravenous heparin. The cannulae are inserted, connected to the appropriate limbs of the ECMO circuit. ECMO support is initiated. Titration The blood flow is increased until respiratory and hemodynamic status is stable, then the blood flow is maintained at that rate. Maintenance Frequent assessment and adjustments are facilitated by continuous venous oximetry, which directly measures the oxyhemoglobin saturation of the blood in the venous limb of the ECMO circuit. Complications • • • • • • • • • • • • Fatal Sepsis Massive hemorrhage (Approximately 50% of reported deaths) Thromboembolism (devastating with VA ECMO than VV ECMO) Pericardial tamponade (from blood or air) Tension pneumothorax or hemothorax Drug effects (alteration in serum concentration of drugs due to increased volume of distribution) Cannula related Cx (vessel perforation with hemorrhage, arterial dissection, distal ischemia, and incorrect location). HIT (If suspected, the heparin infusion is usually replaced by a non-heparin anticoagulant) Veno-Arterial specific complications (LL Ischemia, compartmentsyndrome) Pulmonary hemorrhage Cardiac thrombosis (due to stasis when LV output is not maintained) Metabolic Cx (imbalances in ph, sodium, potassum, Ca & sugar) Critical care challenges in ECMO patient , CACCN, Sep 2008 Special considerations: Blood flow • Near-maximum flow rates - during VV ECMO to optimize oxygen delivery. In contrast, the flow rate used during VA ECMO must be high enough to provide adequate perfusion pressure and venous oxyhemoglobin saturation but low enough to provide sufficient preload to maintain left ventricular output. • pO2 persist low at high FiO2, and Hct is > 35%, flow is increased in increments of 20 cc/min. Left ventricular monitoring • Left ventricular output is rigorously monitored during VA ECMO because left ventricular output can become worse. Diuresis • Since most patients are fluid-overloaded when ECMO is initiated, aggressive diuresis is warranted once the patient is stable on ECMO. • Ultrafiltration can be easily added to the ECMO circuit if patients are unable to produce sufficient urine for diuresis. Fluid management Excluding blood products, 80 – 100 ml/kg/day of volume is generally given Special considerations contd… ACT • Hourly ACT monitoring is mandatory as patient is on heparin infusion & Heparin is titrated according to the ACT levels. The desired ACT levels for a patient on ECMO support is 180-200 sec Hemoglobin & hematocrit levels • The hemoglobin levels should be maintained at least above 10 gm/dl, so as to ensure adequate oxygen carriers. Hct >35 ABG, lactate, glucose or VBG When metabolic acidosis persist the flow is reduced Venous Oximetry • The venous saturation levels displayed on the monitor reflects the oxyhemoglobin saturation at the venous end of the ECMO circuit. Platelets - monitored very closely; their levels must not be too high nor too low. Platelet levels above 100,000/ mm3 is acceptable. PT , Fibrinogen level >150 Electrolytes and ionized calcium Albumin -20% Albumin considered when serum albumin < 2.5. Nursing Implications: Infection Control • All pre-existing invasive lines should be changed prior to starting therapy. Maintain asepsis while handling lines. Anti-coagulation • Despite the utilisation of heparin coated circuits anti-coagulation remains an essential aspect of the therapy. Immobility • Due to the patient’s acuity and the risk of catheter dislodgement, the patient on ECMO will have significantly restricted mobility. Pressure Gradient • The gradient between the pre and post oxygenator pressures indicates the amount of pressure required for blood to pass through the oxygenator. An increasing gradient indicates that higher pressures are needed for the blood to pass. Hourly Assessment • HR, SBP, MAP • Neuro-Vascular Observations to cannulated limbs • Urine Output • Core Temperature • ET CO2 • Routine Ventilation Observations Regular Assessment • 3/24 CVP • 3/24 neurological assessment • Nursing assessment of sedation requirements to prioritize the protection of cannula & circuit integrity. Pre & Post Oxygenator Blood gases ACT 2 hourly General Nursing Care 1. Pressure Area Care and Patient Positioning Log rolling with a designated staff member will be allocated to ensure circuit and cannula safety during the turn. This person should direct the turn. ECMO therapy is associated with fluid shifts and the patient will develop edema, positioning should be aimed to minimize its effect. 2. Gentle care with regard to anticoagulant therapy 3. Dressing changes when there is a significant amount of exudate or if the dressing is no longer secure. Prevent accidental dislodgement Always pull the dressing off towards the insertion site. This will minimize the risk of dislodging the cannula. Nursing Implications 4. Drug levels ECMO will effect drug functioning. Drugs known to be effected by sequestration include Fentanyl, Propofol and Midazolam. There is a drop in serum concentration. This can increase the amount of medication required to effectively sedate the patient. However the effect can reverse over time. 5. Circuit management Ensure the circuit is safely positioned and unlikely to be kinked or trapped in surrounding equipment. Assess the flow by auscultation. Assess the circuit for blood leakage Kicking’ is a sign of inadequate access. Look for any kinks or compression of the cannula or circuit. OR inadequate blood flow should be suspected and the ECMO ‘Kicking’ is indicative of turbulent blood flow which will accelerate the development of clot formation and hemolysis. A reduction in the pump speed or vascular filling may be required ECMO Emergencies - Rare but potentially catastrophic 1. Pump Failure – Call for help, Clamp line and stop pump, Re-engage pump head, Establish the cause and correct if possible 2. Air Embolism in circuit – Call for help, clamp, head down 3. Decannulation • • • Call for help, Clamp circuit, Apply pressure to the cannulation site) Assign someone to manage the clinical status of the patient, Apply rescue ventilation settings Initiate resuscitation as required), 4. Cardiac Arrest – ACLS Nursing Documentation ECMO in Neonates: Indications: • Associated with primary pulmonary hypertension (idiopathic PPHN, meconium aspiration syndrome, respiratory distress syndrome, group B streptococcal sepsis, and asphyxia) • Congenital diaphragmatic hernia (CDH) Selection criteria • Gestational age of 34 wks or more • Birth weight of 2000 g or higher • No significant coagulopathy, intracranial hemorrhage (grade 1 intracranial hemorrhage) • Mechanical ventilation for 10-14 days or less • Reversible lung injury • No major untreatable cardiac malformation • Failure of maximal medical therapy ECMO in Pediatrics • Low cardiac output • Pulmonary vaso-reactive crisis following repair of congenital heart defect leading to severe hypoxemia, low cardiac output, or both • Rarely, as a bridge to cardiac surgery in patients with serious endorgan damage OR as a bridge to cardiac transplant • Possibly, as a bridge to recovery in temporary cardiomyopathy secondary to renal failure, myocarditis, and burns OutcomesAccording to the indication for the ECMO: Acute respiratory failure - With acute respiratory failure use of ECMO has been shown to improve survival rates. The reported survival rates range from 50-70 percent. (in observational and uncontrolled clinical trials). Cardiac failure – Veno-arterial (VA) ECMO is a bridge to further therapy, either a ventricular assist device, transplant or recovery. Survival rates in adults, ECMO survival rates are increased from 35% to 66%. ECMO has yet to have proven survival benefit in adults with ARDS. Rodriguez-Cruz E. ‘Extracorporeal membranous oxygenation’ Medscape MedPulse. Aug 27,2013. SICU – MICU, DH Project: • A teaching for the ICU nurses at DH (in May 2014) during the unit meeting, which ensured maximum attendance. • All about ECMO, its care and followed by a discussion with the perfusionists post lecture. • This was repeated in April 2015 at ECCC, Dubai 2015 • This not increased the nurses’ knowledge and confidence at bedside and but also their cooperation with the perfusionists. • They became more competent in caring for these patients. Discharge outcome in Adults treated with ECMO Single-center, retrospective review of all 212 adult patients treated with ECMO from 2005 through 2009. cardiac (n=126) or respiratory (n = 86) failure. Mean age was 51 (SD, 14.5)years; support duration was 135 (SD, 149) hours. Survival to discharge was 33% overall; 50% for respiratory indication and 21% for cardiac indication patients. Patients with poor outcomes were older (53 vs 47 years, P = .007), For respiratory patients, poor outcome was associated with more ventilator days before ECMO (poor, 6 vs good, 3; P = .01) Conclusions Patients with respiratory indications for ECMO experienced better survival than did cardiac patients. Increasing age was associated with poor outcome. Complications, regardless of indication, were common and associated with poor outcome. (American Journal of Critical Care. 2014; 23:365-377) Future: • Applications for ECMO may expand in the future to include percutaneous temporary left ventricular assistance and low flow ECMO for CO2 removal (ECOOR). In addition, new technologies will improve the simplicity and safety of ECMO, including new oxygenators, pumps, and surface coatings. • A recent study showed that a factor XIIa inhibitory antibody provides thromboprotection in extracorporeal circulation without increasing bleeding risk. • ECMO use on cadavers can increase the viability rate of transplanted organs. REFERENCES: • American Journal of Critical Care. “Discharge outcome in Adults treated with ECMO” 2014; 23:365-377 • ECMO Program website, University of Michigan, Pediatric Surgery retrieved from http://surgery.med.umich.edu/pediatric/research/interests/ecmo.shtml • http://emedicine.medscape.com/article/1818617-overview • E. Joubert – Huebner ‘ECMO Management Clinical Guide’ Chief Perfusionist^Heart Center Eppendorf, Hamburg retrieved from http://www.cardiac-ecc.com/ECMO_Management_Lecture_5.pdf • Extracorporeal Life Support Organization (ELSO) - www.elso.org • Royal Adelaide Hospital ‘ECMO-Nursing care & Responsibilities’ 1st edition July 2010 retrieved from file:///C:/Users/Thomas/Downloads/ECMO%20Nursing%20care%20%20r esponsibilities%255b1%255d.pdf • Canadian Association of critical care nurses “Critical Care Challenges in ECMO patient” 2008, 19;2. View publication stats