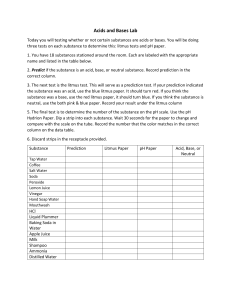
NAME: __________________________________ Use of Indicators to Identify Acids and Bases Purpose: Demonstrate that the pH scale (0-14) is used to measure acidity and classify substances or solutions as acidic, basic, or neutral Background:Many common household products are either acids or bases. Today you will be utilizing various indicators to determine if several common substances are acids, bases, or neutral. Materials: Baking powder, baking soda, vinegar, milk, bleach, dish detergent, soda, bubble solution, apple juice, antacids dissolved in water, ketchup, laundry detergent, pineapple juice, Safety precaution: Please wear goggles and aprons to prevent any splashing. Although these are common household items, they can be eye irritants. Some items may stain clothing. Procedure: There are two solutions at six different stations around the lab. At each station you will dip one strip of red litmus paper and one strip of blue litmus paper into each solution and record the results. After you have completed your testing, please discard the used litmus papers into the provided containers. For each of the following substances, please write down if the solution is acidic or basic and the approximate pH. In addition indicate the Molar Concentration of H+ ion. For example, a pH of 7 would have a Molar Concentration of 1x10-7 M or 0 .0000001 mol/L. 1. Baking powder = 2. Baking soda = 3. Vinegar = 4. Milk = 5. Bleach = 6. Dish Detergent = 7. Soda = 8. Bubble Solution = 9. Apple Juice = 10. Antacids dissolved in water = 11. Ketchup = 12. Laundry detergent = 13. Pineapple juice = 1. Could any of these substances be conductors of electricity? Why? 1. What is the pH scale? How are acids, bases and neutral substances numerically classified on this scale? 2. When would a solution be neutral? How would the ion concentration be different in an acid or base? 3. If one acid has a pH of 1 and a second acid has a pH of 3, how much more acidic is the first substance? 4. If one base has a pH of 11 and a second base has a pH of 14, how much more alkaline is the second substance? 5. On a separate piece of paper draw a replica of the pH scale. Mark all twelve substances on this pH scale and label appropriately. Acid Base Indicators Lab Objective- to determine the pH range and the colors associated with several indicators Materials- Procedure1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 6-50mL beakers Several disposable pipets Indicators Litmus paper blue Litmus paper red Phenolphthalein Universal Indicator Bromocresol Green Bromothymol Blue Methyl Orange Spot Plates Acids and Bases pH 0-2 pH 3 pH 4 pH 5 pH 6 pH 7 (water) pH 8 pH 9 pH 10 pH 11 pH 12 Wear safety goggles at all times. (Acids and Base can be harmful if not handled properly). Clean and dry all glassware and spot plates. Obtain the 5 liquid and 2 paper indicators from you teacher. Label each solution. Place a white sheet of paper under the spot plate. Each station has a different pH solution. Record the solution you are using. Using a pipet add 3-4 drops of the solution to 7 separate wells of the spot plate. Dip the paper indicator into the solution and record your observation on the data table. For liquid indicators use about 2 drops, then record the data. Rinse out and dry the spot plate. Move to the next station when instructed by your teacher. Repeat steps 5 –9 for each solution. Data and Observations- Complete the table. pH Litmus Blue Litmus Red Universal Phenolphtalein Indicator Bromocresol Green 0-2 3 4 5 6 7 8 9 10 11 12 Color change range Questions1. Determine the pH range for the color change for each indicator. 2. What color does litmus blue turn at a pH of 3? 3. What color does methyl orange turn in a pH of 11? 4. What color does phenolphthalein turn in the presence of a base? 5. A base will turn which indicator yellow? 6. A colorless solution using phenolphthalein indicates what? Bromothymol Blue Methyl Orange Conclusion-