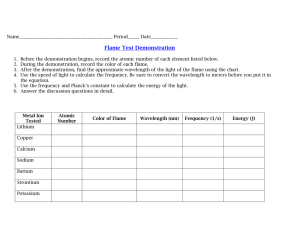
INTRO TO SPECTROSCOPY Spectroscopy – study of interactions between the matter and electromagnetic radiations. Types: Atomic Spectroscopy – concerned with the interactions of electromagnetic radiations with atoms. Molecular Spectroscopy - concerned with the interactions of electromagnetic radiations with molecules. Methods: - These bands are arranged in the increasing order of wavelength. Classification: Line Spectra – spectrum obtained by interaction of electromagnetic radiations with atoms. - Consist of sharp well-defined lines that correspond to a definite frequency. Band Spectra – spectrum obtained by interaction of electromagnetic radiations with molecules. - Non – Destructive Consist of different bands just as visible spectrum Destructive Electromagnetic Radiation – is an oscillating electric and magnetic disturbance that spreads as a wave through empty space, the vacuum. Characteristics: Wavelengths – distance between two crest or troughs. Frequency - number of waves which can pass through a point in one second. Wave Number – reciprocal of wavelength - Total number of waves which can pass through a space of 1 cm Continuous Spectrum – if one color merges into another without a gap Discontinuous Spectrum – if one color will not merge into another Absorption Spectrum - if electromagnetic radiations are passed through a substance, the dark pattern of lines that are obtained corresponding to wavelengths absorbed. Emission Spectrum - if electromagnetic radiations are passed through a substance, the pattern of lines recorded after the emission of the absorbed wavelengths. Amplitude – measure of electric or magnetic field strength at a maximum point in the wave Electromagnetic Spectrum – arrangement of electromagnetic waves in the order of the increasing wavelengths or decreasing frequencies. Period – time in seconds it takes for successive maxima or minima to pass a point in space Absorption – light is absorbed by an atom, ion or molecule taking it to a higher energy state Dispersion of Light – when white light passes through a prism, it is split up into seven colors which correspond to definite wavelengths. Absorption Process – light is absorbed by a substance only when the energy corresponds to some energy need or transition of a substance Spectrum – series of color bands obtained by dispersion of light. Modes: Velocity – distance travelled by a wave in 1 sec. Rotational – occurs in the far IR and microwave region - Changes in the energy of a molecule as it rotates about a center of gravity Vibrational - changes in the average separation of nuclei - Occurs in the mid and far IR regions -log (T) = abc A = abc - Triplet state – spins are parallel Emission – release of a photon by an atom, ion or molecule, taking it to a lower energy level Methods in which the stimulus is usually heat or electrical energy Chemiluminescence – excitation of analyte by a chemical reaction Photoluminescence – emission of photon is measured after absorption Could be fluorescence phosphorescence -log (P/P0) = abc Relationship between A and T Singlet State – state in which spin QNs are opposite - log (P/P0) = abc = A A= log T Electrical - Formula Relationships: or - - Measuring absorbance - Fluorescence – initial absorption of a photon followed by the emission of a second photon - Takes place rapidly and complete in about 10 – 5 secs from the time of excitation - Must be done at the wavelength if maximum absorbance This is because the point of maximum response results to better sensitivity and lower detection limits and reduces possibility of committing errors during measurement Most absorbing species will only give a linear response over a certain range Errors that could occur: Phosphorescence - like fluorescence but there is a delay before emission. Delay can take a few more seconds or hours - Transmittance – is the measured light that passes through the solution (T = P/P0) - Absorbance – governed by Beer Lambert’s law which states that the amount of light absorbed by a solution is an exponential function of concentration and path length of the solution. Absorbance has direct relationship with concentration Transmittance is the one directly measured Some instruments give both transmittance and absorbance information, but transmittance is the preferred parameter As the absorbance of the solution increases, the transmittance decreases At low concentration, a small change in concentration can result in a large change in %T At high concentration changes %T are very small Solution: stay in the range of 80 -20 %T to minimize measurement errors Limitations of Beer Lambert’s Law - - - - Chemical: occurs when the absorbing species is involved in an equilibrium equation Instrumental: Strictly valid for purely monochromatic radiation that is for radiation consisting of only one wavelength. Presence of stray radiation that arises from imperfections within the wavelength selector that allows extraneous light to “leak” into the instrument. Stray radiation adds an additional contribution, P stray to the radiant power reaching the detector. For smaller concentrations of analyte, P stray is significantly smaller that Po and P, and the absorbance is unaffected. At higher concentrations however, P stray is no longer significantly smaller than P and the absorbance is smaller than expected. The result is a negative deviation of Beer’s law A = log (Po + P stray / P + P stray) Emission – light produced is directly proportional to the concentration of species being measured - C = kI, k (proportionality constant), I (intensity of light), C (concentration) Quantitative analysis of single analyte – concentration of analyte is determined by measuring the absorbance of the sample and applying Beer’s law using any of the standardization methods such as normal calibration curves and method of standard additions. - The absorbance of a solution at any wavelength is the sum of absorbances of all species in the solution. reactants by preparing solutions containing different mole fractions of one reactant. - Y = nL/nM = XL/XM = XL/1-XL Job’s method Mole ratio method – procedure of determining the stoichiometry between two reactants by preparing solutions containing different mole ratios of two reactants - - Absorbance is monitored at a wavelength at which metal ligand complex absorbs A plot of absorbance as a function of metal ligand to metal mole ratio has two linear branches that intersect at a mole ratio corresponding to the formula of the complex Slope Ratio method – procedure of determining the stoichiometry between two reactants by measuring the relative change in absorbance under conditions when each reactant is a limiting reagent - For weak complexes Prepare two sets of solutions: series of M solutions (varied amount of M with excess of L) and series of L solutions (varied amount of L with excess of M) UV VIS Spectroscopy The wavelength and amount of light that a compound absorbs depends on its molecular structure and the concentration of the substance used. Concentration dependence follows the Beer’s law: A=eBc A (absorbance) =log10 (P0/P) Stoichiometry of Metal-Ligand Complex Methods of determination: Method of continuous variations – procedure of determining the stoichiometry between two e (molar absorbtivity with units of L mol-1 cm-1) B (path length of the sample, cm) c (concentration of compound in solution, mol/L) Spectrophotometer - is a device used to measure the transmittance or absorbance of a sample as a function of the wavelength of the electromagnetic spectrum. The key components of UV-visible spectrophotometers are: - - - A source which generates a broad band of electromagnetic radiation (UV and visible) A dispersion device (monochromator) which selects a particular wavelength or wavelengths A sample cell One or more detectors to measure the intensity of radiation Atomic Spectroscopy Optical Spectrometry – elements present in a sample are converted to gaseous atoms or elementary ions by atomization process and the UV-vis absorption, emission or fluorescence of atomic species in the vapor is then measured Mass Spectrometry – samples are also atomized but the gaseous atoms are converted to positive ions and separated according to their mass-tocharge ratios and quantitative data are obtained by counting the separated ions X-ray Spectrometry – does not require atomization because most elements are largely independent of their chemical composition in a sample thus quantitative results are based on direct measurement of the fluorescence, absorption or emission spectrum of a sample. Principle: Samples vaporized at 2000 – 8000 K decompose into atoms and concentration of these atoms in the vapor are measured by emission or absorption of characteristic wavelengths of radiation - In atomic absorption, atoms absorb part of the light from the source and the remainder of the light reaches the detector Atomic emission comes from atoms that are in an excited state because of the high thermal energy of the flame To observe atomic fluorescence, atoms are excited by an external lamp or laser. An excited atom can fall to a lower state and emit radiation. Characteristics of Atomic absorption: - Absorption of sharp lines from hollow cathode lamp High sensitivity Ability to distinguish one element from the other in a complex sample Ability to perform simultaneous multielement analysis Easiness in the analysis of samples Distinguishing Features: - - - Types: - Emission of thermally populated excited state Fluorescence following absorption of laser radiation - Analysis is limited to the elements (metals) Sample separation schemes for atomic spectroscopy usually place the metals in water solution. It must have a means of converting metal ions into free gas phase ground state atoms (atomization) in order to measure them The sample container used is the source of the thermal energy needed for the conversion of ions in solution to atoms in the gas phase (atomizer) Spectral line sources are used as light sources in atomic absorption instrument and several atomic emission techniques require no light source at all apart from the thermal energy source The wavelengths used for quantitation are well known and do not require the analyst to ever first measure the absorption or emission spectra Optical Spectra – consist of narrow spectral lines since only electronic transition occurs - - Types: Emission Spectra – analyte atoms are excited by external energy in the form of heat or electrical energy - Transition to or from ground state is called resonance transition and the resulting spectral line is called resonance line Absorption Spectra – external source of radiation impinges on the analyte vapor - - Doppler Broadening – during atomization, species may move towards or away from the detector The radiation is absorbed by the analyte atoms and promote them to excited states - Fluorescence Spectra – same external source as absorption - - Radiant power of fluorescence is measured at right angles to the source Resonance fluorescence happens when atomic fluorescence measured at the same wavelength as the source of radiation - - - Atomic spectra have narrow lines and anything that causes broadening of the lines can cause problems Narrow lines are highly desirable for both atomic and emission spectra because they reduce the possibility of interference due to overlapping lines Four Sources of Broadening: Natural broadening due to uncertainty effect Doppler shift in the resulting line occurs Spectral lines that are 1 – 5 nm wide result to 100 times magnification Nothing can be done except to recognize that it occurs Causes: Atomic Line width – the width of atomic lines is quite important in atomic spectroscopy - The line width of the source must be narrower than the line width of the atomic vapor for Beer’s law to be obeyed. Line width is governed by quantum mechanical effect called Heisenberg uncertainty principle stating that the shorter the lifetime of the excited state, the more uncertain is its energy relative to the ground state Typical radiative lifetimes of the atoms are on the order 10-8 s which lead to the natural line widths on the order of 10-5 nm When atom moves toward a photon detector it emits radiation, the detector sees wave crest more often and defects radiation of higher frequency When atom moves away from photon detector and emits radiation, the detector sees wave crest less frequent and detects radiation of lower frequency Pressure broadening - arises from the collision of the sample atoms with other species causing some energy to be exchanged - - Amount of broadening increases with the concentration of the collision partners Has greater effect a temperature increase Electric and magnetic effect Types of Atomizers used for Absorption Spectroscopy: created a plume of a particulate and vaporized sample is transported by an inert gas Continuous atomizer – samples are introduced in a continuous manner Flame Atomization – utilize large flames as the atomizer in which the sample is drawn into the flame via vacuum mechanism. The light beam for the absorption measurement is directed through the width of the flame right to left or left to right depending on the instrument orientation and measured - - Samples are frequently introduced into plasmas or flames via nebulizer producing a mist of spray Samples maybe introduced via flow injection or HPLC or separate converted to vapor Discrete Atomizer – samples are introduced using a device such as syringe or autosampler - - Methods for introduction of sample: Pneumatic Nebulizer – the liquid sample is drawn by a capillary tube by a high-pressure stream of gas flowing around the tip of the tube and the process is called aspiration Ultrasonic Nebulizer – liquid sample is drawn by a capillary tube by a high-pressure stream of gas flowing around the tip of the tube and the process is called aspiration the sample is pumped onto the surface of a piezoelectric crystal. It produces denser and more homogenous aerosols compared to the pneumatic nebulizer Electrothermal vaporizers – an evaporator in which argon flows to carry the vaporized sample into the atomizer Hydride generators – use to carry highly toxic species in low concentration levels into an atomizer as a gas. Direct sample insertion – sample is physically placed in atomizer Electrothermal vaporizers (flameless) – sample is heated conductively on or in a graphite or tantalum rod or boat and sample is carried by an inert gas Arc, spark or laser ablation – sample discharge interacts with the surface of a solid sample and - Flame atomizer will usually have a long narrow burner (premix burner) that serves as sample path Nebulizer controls sample flow, producing a mist Mixing chamber assures the sample mixes with the oxidant and fuel prior to entry to the flame Process: Process occurring in the flame: - combination works best with a given element Air-acetylene flames are the most commonly used Radiation Source: Hollow Cathode Lamp – source produces emissions specific for the element used to construct the cathode - Cathode must be capable of conducting a current for it to work Will only produce emission lines for the cathode element Limitation: metal is too volatile, good cathode can’t be produced, metal may not be a good conductor Mechanism: Premix burner – components of the flame (fuel, oxidant and sample solution) are premixed as they take a common path to the flame - Fuel and oxidant originate from pressurized sources and their flow the burner is controlled at an optimum rate by flow control mechanisms that are part of the overall instrument Laminar flow burner – provides a relatively quiet flame and long path length Fuel and Oxidant – all flames require these - - - To achieve desirable sensitivity, hotter flame is desirable for quantitative analysis to sufficiently atomize sample Aside from flame temperature, burning velocity should also be considered because high burning velocity decreases the completeness of the atomization and therefore actually lowering the sensitivity The choice is made based on which flame temperature-burning velocity Electrodeless Discharge Lamp – has no anode or cathode - Small, sealed quartz tube containing the metal or metal salt and some argon at low pressure wrapped with a coil for the purpose of creating a radio frequency field Radiation Source Modulation – used to eliminate interferences caused by the emission of radiation by the flame Chopper – used to provide signal modulation - in conjunction with a lock – in amplifier - - - It is not practical to have separate cells, so the light is simply split, with half being sent around the atomization source This reduces some noise from the atomization source and accounts for instrumental variations Types: rotating vane (b), rotating disk (a), oscillating tuning fork (c) where they cause periodic interruption of light beam Optical Path – light source then flames then monochromator then detector. Monochromator is placed after flame because flame is necessarily in an open area and is surrounded with room light. This ensures that light from the room will not leak to the detector and the flame emissions will be eliminated - Maybe single or double beam Single Beam Double Beam – primary function is to eliminate problems due to source drift and fluctuation - Side advantage is that source warm up time is eliminated since changes in intensity during warm up are immediately compensated and thus very rapid changeover of lamps in automated instruments is possible Monochromator and Detector – high resolution, holographic grating is used to resolve the lines. It is not designed to be used in scan mode - Typical detector is photomultiplier tube Method of background correction since signal modulation simply accounts for flame flicker and instrument variations but not accounts for background absorption of emission Interferences in AAS Spectral Interferences – due to substances in the flame that absorb the same wavelength as the analyte, causing the absorbance measured to be high - Absorption due to the presence of light absorbing molecules in the flame and light dimming due to the presence of small particles in the flame are much more common spectral interferences these are called background absorption Methods of Correction: Zeeman Effect – magnetic field is applied to the sample. When an atomic vapor is exposed to a strong magnetic field, there is a splitting of the atom’s electronic energy levels. This essentially moves the absorption away from emission lines. Using this method, we can measure at a fixed and narrow wavelength. At regular intervals, we simply move our sample component out of the way. This allows us to directly measure the background Deuterium correction – continuous source correction method wherein light from both AA source lamp and D2 lamp alternately pass through the sample. Because spectral width is significantly larger than AA source line, the D2 lamp send a much broader band of light to the sample - HC (narrow band width) D2 (larger band width) Limitations: either under correction or over correction can occur based on the sample, background may vary around line, composition of background can differ based on position in flame (requires good alignment of HC and D2 lamp), D2 output is not very good at greater than 350 nm Chemical Interferences – results of problems with the sample matrix - Correction: matrix matching (matching the matrix of all of standards as these solutions are prepared with that of the sample so that the same matrix effect is seen in all solutions measured and therefore becomes inconsequential, standard addition method Practical Matters and Applications Slits and Spectral lines Linear and non-linear standard curves Hollow cathode lamp current Lamp alignment Aspiration rate Burner head position Fuel and oxidant source and flow rates