Atomic Spectroscopy Test Questions - College Chemistry
advertisement

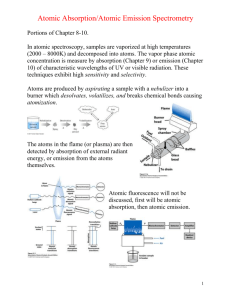
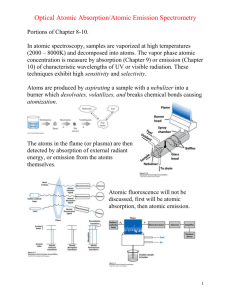
Harris QCA 8e Chapter 20 1. In atomic emission spectroscopy, it is desirable to have a high concentration of the element in the form of (a) atoms in the ground state. (b) atoms in the excited state. (c) ions. 2. A rich flame in atomic absorption spectroscopy would (a) decrease the concentration of metal oxides in the flames. (b) be hotter than a lean flame. (c) be preferred for refractory elements. 3. Compared to a flame, a graphite furnace (a) has lower sensitivity. (b) requires less sample. (c) operates at a higher temperature. 4. The temperature of a graphite furnace is (a) isothermal at approximately 2500 °C. (b) programmed to move at a constant rate from room temperature to approximately 2500 °C. (c) programmed to move in steps from room temperature to approximately 2500 °C. 5. For a given element, the atomization method that can detect the lowest concentration is (a) flame. (b) furnace. (c) plasma. 6. A matrix modifier such as Mg(NO3)2 is used to (a) prevent premature evaporation of the analyte. (b) prevent ionization of the analyte. (c) prevent formation of metal oxides. 7. A higher temperature in the atomizer would (a) be a benefit in atomic absorption. (b) be a benefit in atomic emission. (c) have a negative effect on both atomic absorption and atomic emission. 8. A sample containing an unknown concentration of Ca2+ gave an absorbance of 0.250 in atomic absorption. "Spiking" the sample with enough Ca2+ to increase the concentration by 0.200 M, without significantly diluting the sample, gave an absorbance of 0.450. The concentration of Ca2+ in the original sample was (a) 0.111 molar. (b) 0.077 molar. (c) 0.250 molar. 9. Doppler broadening is due to (a) movement of atoms toward and away from the source. (b) collisions between atoms in the flame. (c) increasing line width of the incident light. 10. Hollow cathode lamps (a) are used for atomic absorption and ICP-MS. (b) apply a high voltage between anode and cathode in an evacuated lamp. (c) differ for each element studied. 11. The general types of atomic spectroscopy are (a) absorption, flame, and emission. (b) absorption, phosphorescence, and emission. (c) absorption, fluorescence, and emission. 12. The optical spectra of liquids and solids is typically (a) ~1000 nm, and the spectra of gaseous atoms is ~0.001 nm. (b) ~100 nm, and the spectra of gaseous atoms is ~0.0001 nm. (c) ~0.0001 nm, and the spectra of gaseous atoms is ~100 nm. 13. The Boltzmann distribution describes (a) the relative error of different states at thermal equilibrium. (b) the relative populations of different states at thermal equilibrium. (c) the relative populations of different states at chemical equilibrium. 14. The Heisenberg uncertainty principle says that (a) the shorter the lifetime of the excited state, the more uncertain is its energy. (b) the longer the lifetime of the excited state, the more uncertain is its energy. (c) the shorter the lifetime of the ground state, the more uncertain is its energy. 15. Atomic spectroscopy must provide background correction to (a) distinguish interferent signal from absorption, emission, and optical scattering of the sample matrix, the flame, plasma, or white-hot graphite furnace. (b) distinguish analyte signal from absorption, emission, and optical scattering of the sample matrix, the flame, plasma, or white-hot graphite furnace. (c) distinguish analyte signal from absorption, emission, and optical scattering of the sample.