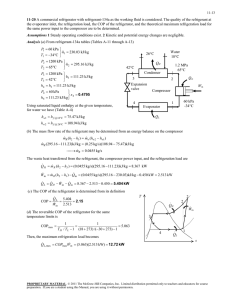
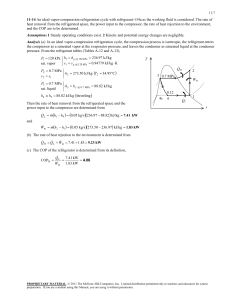
Refrigeration & Air Conditioning: Principles & Applications
advertisement

REF & AC I What is refrigeration Refrigeration is a process of removing heat from a low-temperature reservoir and transferring it to a high-temperature reservoir. The work of heat transfer is traditionally driven by mechanical means, but can also be driven by heat, magnetism, electricity, laser, or other means. Principle of refrigeration •Liquids absorb heat when changed from liquid to gas •Gases give off heat when changed from gas to liquid. What is the principle behind the working of a refrigerator? A refrigerator does not cool items by lowering their original temperatures; instead, an evaporating gas called a refrigerant draws heat away, leaving the surrounding area much colder. Refrigerators and air conditioners both work on the principle of cooling through evaporation. Purpose of refrigeration • To keep food cold/ Food storage • Refrigerators maintain medication temperatures of sensitive drug • Most commonly used to achieve a more comfortable interior environment, typically for humans and animals • Used to cool/dehumidify rooms filled with heat-producing electronic devices, such as computers and electronic devices, and even to display and store some delicate products, such as artwork Refrigeration HEAT TRANSFER. ... Heat transfer is the method by which heat flows. This is another basic principle of refrigeration. The evaporator transfers heat into the refrigerant; the refrigerant transfers this heat to the condenser; the condenser transfers the heat to a cooling medium (such as water or outside air) Refrigeration cycle: The vapor compression refrigeration cycle is a common method for transferring heat from a low temperature to a high temperature. The purpose of a refrigerator is the removal of heat, called the cooling load, from a low-temperature medium. What is the unit of refrigeration? A ton of refrigeration (TR), also called a refrigeration ton (RT), is a unit of power used in some countries (especially in North America) to describe the heat-extraction capacity of refrigeration and air conditioning equipment. Refrigerants, are chemical compounds that are alternately compressed and condensed into a liquid and then permitted to expand into a vapor or gas as they are pumped through the mechanical refrigeration system to cycle. Essential requirements for refrigeration • Size • Space • Type • Reliability • Power consumptions Difference between refrigerator and air conditioning Refrigerator or cooling technology is a technology branch that deals with phenomena and processes of body cooling. In this sense, cooling means reducing the internal energy of a body by removal of energy, which is manifested by lowering of its temperature while Air conditioning is the process of changing the air condition by removing the heat and moisture in order to achieve a more comfortable inner environment. The goal of this process is usually to distribute air-conditioned air in different indoor spaces in order to achieve certain comfort and air quality. Air conditioners often use a fan to distribute the conditioned air to an occupied space such as a building or a car to improve thermal comfort and indoor air quality. Air conditioning Air conditioning is a combined process that performs many functions simultaneously. It conditions the air, transports it, and introduces it to the conditioned space. It provides heating and cooling from its central plant or rooftop units. It also controls and maintains the temperature, humidity, air movement, air cleanliness, sound level, and pressure differential in a space within predetermined limits for the comfort and health of the occupants of the conditioned space or for the purpose of product processing. The term HVAC&R is an abbreviation of heating, ventilating, air conditioning, and refrigerating. The combination of processes in this commonly adopted term is equivalent to the current definition of air conditioning. Because all these individual component processes were developed prior to the more complete concept of air conditioning, the term HVAC&R is often used by the industry Air conditioning Most air conditioning systems perform the following functions: 1. Provide the cooling and heating energy required 2. Condition the supply air, that is, heat or cool, humidify or dehumidify, clean and purify, and attenuate any objectionable noise produced by the HVAC&R equipment 3. Distribute the conditioned air, containing sufficient outdoor air, to the conditioned space 4. Control and maintain the indoor environmental parameters – such as temperature, humidity, cleanliness, air movement, sound level, and pressure differential between the conditioned space and surroundings — within predetermined limits Air conditioning systems can be classified according to their applications as (1) comfort air conditioning systems and (2) process air conditioning systems Application of refrigeration Domestic refrigeration Domestic refrigerator (also household refrigerator),an appliance that is used for the short-term preservation of food products in the home by means of refrigeration. Depending on the type of refrigerating machine used, domestic refrigerators are classified as compression-type, absorption-type, and thermoelectric. The first domestic refrigerators, which used vapor-compression machines, appeared in 1910 in the USA. Absorption-type domestic refrigerators were first produced in 1925 in Sweden. The first thermoelectric domestic refrigerators were manufactured in the second half of the 1950’s. In the USSR, the first compression-type domestic refrigerators (the KhTZ-120) were produced in 1939, the first absorption-type domestic refrigerators (the Gazoapparat) were manufactured in 1945, and the first prototypes of a thermoelectric refrigerator, which uses thermoelectric cooling, were produced in 1951. The mass production of Soviet compression-type domestic refrigerators (the ZIL) began in 1951. Domestic refrigerators A domestic refrigerator is a metal cabinet with a built-in hermetically sealed refrigerating unit. Inside the cabinet is a cold chamber with shelves for storing products. Heat insulation is placed between the walls of the cold chamber and the case of the refrigerator. The air in the cold chamber is cooled by means of heat transfer between the air and the cold surface of the evaporator. The necessary temperature conditions in the refrigerator are provided by the brief periodic—that is, cyclic—operation of the refrigerating unit, which is switched on by means of a thermal relay. Domestic refrigerators have a storage space of 20 to 800 liters. Domestic refrigerators may be stationary or portable. Stationary refrigerators are classified as freestanding, wall-type, and built-in (that is, built into a kitchen or reception-room furniture unit). Portable models are mainly absorption-type and thermoelectric refrigerators. In addition, absorption-type refrigerators are classified, depending on the heat source, as electric, gas-fired, kerosene-burning, and combination-type. Electric refrigerators are the most widely used. The limited use of gas-fired refrigerators is due mainly to safety considerations and also to difficulties associated with the connection of such refrigerators to a gas distribution system. Kerosene-burning refrigerators are used mainly aboard ships and as portable appliances. Domestic refrigerator Most of the domestic refrigerators manufactured today are of the compression type. Absorption-type refrigerators account for 5–10 percent of the total production. In comparison with compression-type refrigerators, absorptiontype refrigerators are bulkier and heavier, consume more electrical energy (more by a factor of 1.5–2), and have a smaller freezer compartment. Thermoelectric refrigerators are not widely used, since they are expensive and have higher power requirements than compression-type refrigerators Commercial refrigerator Commercial refrigerator means a unit of commercial refrigeration equipment in which all refrigerated compartments in the unit are capable of operating at or above 32 °F (±2 °F). Commercial refrigerator, freezer, and refrigerator-freezer means refrigeration equipment that (1) Is not a consumer product (2) Is not designed and marketed exclusively for medical, scientific, or research purposes; (3) Operates at a chilled, frozen, combination chilled and frozen, or variable temperature; (4) Displays or stores merchandise and other perishable materials horizontally, semi-vertically, or vertically; (5) Has transparent or solid doors, sliding or hinged doors, a combination of hinged, sliding, transparent, or solid doors, or no doors; (6) Is designed for pull-down temperature applications or holding temperature applications; and (7) Is connected to a self-contained condensing unit or to a remote condensing unit. Industrial refrigeration Industrial refrigeration covers a wide range of applications, from high temperature process chilling, through to very low temperature applications such as those used in medical freezers or LNG liquefaction. Transport refrigeration/marine Transport refrigeration is essential in today's society, to preserve and protect food, drugs and medical supplies for people worldwide Trailers, large and small trucks but also containers and rail carriages are refrigerated in order to preserve perishable goods. Smaller units are connected to the motor directly or via an alternator. Bigger units have their own diesel units. Comfort Air conditioning Comfort air conditioning systems provide occupants with a comfortable and healthy indoor environment in which to carry out their activities. The various sectors of the economy using comfort air conditioning systems are as follows: 1. The commercial sector includes office buildings, supermarkets, department stores, shopping centers, restaurants, and others. Many high-rise office buildings, including such structures as the World Trade Center in New York City and the Sears Tower in Chicago, use complicated air conditioning systems to satisfy multiple-tenant requirements. In light commercial buildings, the air conditioning system serves the conditioned space of only a single-zone or comparatively smaller area. For shopping malls and restaurants, air conditioning is necessary to attract customers. 2. The institutional sector includes such applications as schools, colleges, universities, libraries, museums, indoor stadiums, cinemas, theaters, concert halls, and recreation centers. For example, one of the large indoor stadiums, the Superdome in New Orleans, Louisiana, can seat 78,000 people. 3. The residential and lodging sector consists of hotels, motels, apartment houses, and private homes. Many systems serving the lodging industry and apartment houses are operated continuously, on a 24-hour, 7-day-a-week schedule, since they can be occupied at any time. 4. The health care sector encompasses hospitals, nursing homes, and convalescent care facilities. Special air filters are generally used in hospitals to remove bacteria and particulates of submicrometer size from areas such as operating rooms, nurseries, and intensive care units 5. The transportation sector includes aircraft, automobiles, railroad cars, buses, and cruising ships. Passengers increasingly demand ease and environmental comfort, especially for long-distance travel. Process Air Conditioning Systems Process air conditioning systems provide needed indoor environmental control for manufacturing, product storage, or other research and development processes. The following areas are examples of process air conditioning systems: 1. In textile mills, natural fibers and manufactured fibers are hygroscopic. Proper control of humidity increases the strength of the yarn and fabric during processing. For many textile manufacturing processes, too high a value for the space relative humidity can cause problems in the spinning process. On the other hand, a lower relative humidity may induce static electricity that is harmful for the production processes. 2. Many electronic products require clean rooms for manufacturing such things as integrated circuits, since their quality is adversely affected by airborne particles. Relative-humidity control is also needed to prevent corrosion and condensation and to eliminate static electricity. Temperature control maintains materials and instruments at stable condition and is also required for workers who wear dust-free garments. 3. Pharmaceutical products require temperature, humidity, and air cleanliness control. For instance, liver extracts require a temperature of 75°F (23.9°C) and a relative humidity of 35 percent. If the temperature exceeds 80°F (26.7°C), the extracts tend to deteriorate. Refrigeration and Heat pumps cycles The Carnot Engine and Cycle The Carnot cycle was introduced as the most efficient heat engine that can operate between two fixed temperatures TH and TL. The Carnot cycle is described by the following four processes. Process Description 1-2 Reversible isothermal heat addition at high temperature, TH > TL, to the working fluid in a piston cylinder device that does some boundary work. 2-3 Reversible adiabatic expansion during which the system does work as the working fluid temperature decreases from TH to TL. 3-4 The system is brought in contact with a heat reservoir at TL < TH and a reversible isothermal heat exchange takes place while work of compression is done on the system. 4-1 A reversible adiabatic compression process increases the working fluid temperature from TL to TH The Carnot Engine and Cycle We often use the Carnot efficiency as a means to think about ways to improve the cycle efficiency of other cycles. One of the observations about the efficiency of both ideal and actual cycles comes from the Carnot efficiency: Thermal efficiency increases with an increase in the average temperature at which heat is supplied to the system or with a decrease in the average temperature at which heat is rejected from the system. Refrigerator and a heat pump A refrigerator may be defined as a device that operates in a thermodynamic cycle and transfers a certain amount of heat from a body at a lower temperature to a body at a higher temperature by consuming certain amount of external work. Domestic refrigerators and room air conditioners are the examples. In a refrigerator, the required output is the heat extracted from the low temperature body. A heat pump is similar to a refrigerator, however, here the required output is the heat rejected to the high temperature body. A refrigerator or a heat pump that operates on the reversed Carnot cycle is called a Carnot refrigerator, or a Carnot heat pump. The Reversed Carnot Cycle The Carnot cycle is a totally reversible cycle that consists of two reversible isothermal and two isentropic processes. It has the maximum thermal efficiency for given temperature limits, and it serves as a standard against which actual power cycles can be compared. The Carnot heat-engine cycle just described is a totally reversible cycle. Therefore, all the processes that comprise it can be reversed, in which case it becomes the Carnot refrigeration cycle. This time, the cycle remains exactly the same, except that the directions of any heat and work interactions are reversed: Heat in the amount of QL is absorbed from the low-temperature reservoir, heat in the amount of QH is rejected to a high-temperature reservoir, and a work input of Wnet,in is required to accomplish all this. The P-V diagram of the reversed Carnot cycle is the same as the one given for the Carnot cycle, except that the directions of the processes are reversed, as shown in Fig. The reversed Carnot cycle is the most efficient refrigeration cycle operating between two specified temperature levels. Therefore, it is natural to look at it first as a prospective ideal cycle for refrigerators and heat pumps. The reversed Carnot cycle is not a suitable model for refrigeration cycles. TS Diagram for a Reversed Carnot Cycle Since it is a reversible cycle, all four processes that comprise the Carnot cycle can be reversed. Reversing the cycle does also reverse the directions of any heat and work interactions. The result is a cycle that operates in the counterclockwise direction on a T-s diagram, which is called the reversed Carnot cycle. Schematic of a Carnot refrigerator and T-s diagram of the reversed Carnot cycle. TS Diagram for a Reversed Carnot Cycle Consider a reversed Carnot cycle executed within the saturation dome of a refrigerant, as shown in the Fig. The refrigerant absorbs heat isothermally from a low-temperature source at T in the amount of Q (process 1-2), is compressed isentropically to state 3 (temperature rises to T ), rejects heat isothermally to a high-temperature sink at T in the amount of Q (process 3-4), and expands isentropically to state 1 (temperature drops to T ). The refrigerant changes from a saturated vapor state to a saturated liquid state in the condenser during process 3-4. L L H H H L The Reversed Carnot Cycle Here QLis the magnitude of the heat removed from the refrigerated space at temperature TL ,QH is the magnitude of the heat rejected to the warm environment at temperature TH, and Wnet, in is the net work input to the refrigerator. As discussed, QL and QH represent magnitudes and thus are positive quantities. Coefficient of Performance of Refrigerator The efficiency of a refrigerator is expressed in terms of the coefficient of performance (COP), denoted by COPR. The objective of a refrigerator is to remove heat (QL) from the refrigerated space. To accomplish this objective, it requires a work input of Wnet,in. Then the COP of a refrigerator can be expressed as COPR = 𝐷𝑒𝑠𝑖𝑟𝑒𝑑 𝑜𝑢𝑡𝑝𝑢𝑡 𝑅𝑒𝑞𝑢𝑖𝑟𝑒𝑑 𝑖𝑛𝑝𝑢𝑡 = 𝑄𝐿 𝑊𝑛𝑒𝑡,𝑖𝑛 The conservation of energy principle for a cyclic device requires that 𝑊𝑛𝑒𝑡,𝑖𝑛 = 𝑄𝐻 − 𝑄(KJ) 𝐿 Coefficient of Performance of Refrigerator Then the COP relation becomes COPR = 𝑄𝐿 𝑄𝐻 −𝑄𝐿 = 1 𝑄𝐻 𝑄𝐿 −1 Notice that the value of COPR can be greater than unity. That is, the amount of heat removed from the refrigerated space can be greater than the amount of work input. This is in contrast to the thermal efficiency, which can never be greater than 1. In fact, one reason for expressing the efficiency of a refrigerator by another term—the coefficient of performance—is the desire to avoid the oddity of having efficiencies greater than unity. Coefficient of Performance of a Heat Pump The measure of performance of a heat pump is also expressed in terms of the coefficient of performance COPHP, defined as 𝐷𝑒𝑠𝑖𝑟𝑒𝑑 𝑜𝑢𝑡𝑝𝑢𝑡 COPHP= 𝑅𝑒𝑞𝑢𝑖𝑟𝑒𝑑 𝑖𝑛𝑝𝑢𝑡 = 𝑊 𝑄𝐻 𝑛𝑒𝑡,𝑖𝑛 which can also be expressed as COPHP= 𝑄𝐻 𝑄𝐻 −𝑄𝐿 = 1 1−𝑄𝐿 𝑄𝐻 COPHP= COPR + 1 for fixed values of QL and QH This relation implies that the coefficient of performance of a heat pump is always greater than unity since COPR is a positive quantity. COP of Refrigerator and Heat Pump The coefficients of performance of Carnot refrigerators and heat pumps are expressed in terms of temperatures as COPR, Carnot= 1 𝑇𝐻 𝑇𝐿 −1 COPHP, Carnot= 1 1−𝑇𝐿 𝑇𝐻 Notice that both COPs increase as the difference between the two temperatures decreases, that is, as TL rises or TH falls. Example The food compartment of a refrigerator, shown in the Fig, is maintained at 4°C by removing heat from it at a rate of 360 kJ/min. If the required power input to the refrigerator is 2 kW, determine (a) the coefficient of performance of the refrigerator and (b) the rate of heat rejection to the room that houses the refrigerator. Solution The power consumption of a refrigerator is given. The COP and the rate of heat rejection are to be determined. Assumptions Steady operating conditions exist. Analysis (a) The coefficient of performance of the refrigerator is COPR = 𝑄𝐿 𝑊𝑛𝑒𝑡,𝑖𝑛 = 360𝐾𝐽/𝑚𝑖𝑛 1𝐾𝑊 2𝐾𝑊 60𝐾𝐽/𝑚𝑖𝑛 =3 That is, 3 kJ of heat is removed from the refrigerated space for each kJ of work supplied. (b) The rate at which heat is rejected to the room that houses the refrigerator is determined from the conservation of energy relation for cyclic devices, 360𝐾𝐽 60𝐾𝐽/𝑚𝑖𝑛 𝑄𝐻 = 𝑄𝐿 + 𝑊𝑛𝑒𝑡,𝑖𝑛 = + 2 𝐾𝑊 = 480𝐾𝐽/𝑚𝑖𝑛 𝑚𝑖𝑛 1𝐾𝑊 The vapor compression refrigeration cycle is a common method for transferring heat from a low temperature to a high temperature. The above figure shows the objectives of refrigerators and heat pumps. The purpose of a refrigerator is the removal of heat, called the cooling load, from a lowtemperature medium. The purpose of a heat pump is the transfer of heat to a hightemperature medium, called the heating load. When we are interested in the heat energy removed from a low-temperature space, the device is called a refrigerator. When we are interested in the heat energy supplied to the high-temperature space, the device is called a heat pump. In general, the term heat pump is used to describe the cycle as heat energy is removed from the low-temperature space and rejected to the high-temperature space. 34 The performance of refrigerators and heat pumps is expressed in terms of coefficient of performance (COP), defined as Desired output Cooling effect QL COPR Required input Work input Wnet ,in COPHP Desired output Heating effect Q H Required input Work input Wnet ,in Both COPR and COPHP can be larger than 1. Under the same operating conditions, the COPs are related by COPHP COPR 1 Can you show this to be true? Refrigerators, air conditioners, and heat pumps are rated with a SEER number or seasonal adjusted energy efficiency ratio. The SEER is defined as the Btu/hr of heat transferred per watt of work energy input. The Btu is the British thermal unit and is equivalent to 778 ft-lbf of work (1 W = 3.4122 Btu/hr). An EER of 10 yields a COP of 2.9. Refrigeration systems are also rated in terms of tons of refrigeration. One ton of refrigeration is equivalent to 12,000 Btu/hr or 211 kJ/min. 35 The Vapor-Compression Refrigeration Cycle The vapor-compression refrigeration cycle has four components: evaporator, compressor, condenser, and expansion (or throttle) valve. The most widely used refrigeration cycle is the vapor-compression refrigeration cycle. In an ideal vaporcompression refrigeration cycle, the refrigerant enters the compressor as a saturated vapor and is cooled to the saturated liquid state in the condenser. It is then throttled to the evaporator pressure and vaporizes as it absorbs heat from the refrigerated space. The ideal vapor-compression cycle consists of four processes. Ideal Vapor-Compression Refrigeration Cycle Process Description 1-2 Isentropic compression 2-3 Constant pressure heat rejection in the condenser 3-4 Throttling in an expansion valve 4-1 Constant pressure heat addition in the evaporator 36 The P-h diagram is another convenient diagram often used to illustrate the refrigeration cycle. 37 The ordinary household refrigerator is a good example of the application of this cycle. COPR COPHP Q L h h 1 4 Wnet ,in h2 h1 Q H h h 2 3 Wnet ,in h2 h1 38 Example Consider an ideal refrigeration cycle which uses R-12 as working fluid. The temperature of the refrigerant in the evaporator is -20oC and in the condenser it is 40oC. The refrigerant is circulated at the rate of 0.03kg/s. determine the coefficient of performance and the capacity of the plant in rate of refrigeration. For control volume analysed, the thermodynamic model is the R-12 table Compressor T1 known, saturated vapour state fixed P2 known (saturation pressure at T3) From the first law Wnet, in = h1 – h2 From the second law s2=s1 Solution At T3 = 40oC, Pg = P2 = 0.9607MPa From the R-12 tables, h1 = 178.61, s1 = 0.7082 Therefore, s2=s1= 0.7082 So, T2 = 50.8oC and h2 = 211.38 Wnet, in = h1 – h2 = 211.38 – 178.61 = 32.77kJ/kg Expansion valve Inlet state: T3 is known, saturated liquid, state fixed Exit state T4 From first law h4= h3= 74.587 Evaporator Inlet state: State 4 known (as given) Exit state : State 1 known First law qL = h1 – h4 = 178.61 – 74.587 =104.02kJ/kg Therefore, the COPR = qL/Wnet,in =104.02/32.77 =3.17 The capacity of the plant in rate of refrigeration = 104.02 x 0.03 = 3.12kW Example 11-1 Refrigerant-134a is the working fluid in an ideal compression refrigeration cycle. The refrigerant leaves the evaporator at -20oC and has a condenser pressure of 0.9 MPa. The mass flow rate is 3 kg/min. Find COPR and COPR, Carnot for the same Tmax and Tmin , and the tons of refrigeration. Using the Refrigerant-134a Tables, we have kJ h 238.41 1 Compressor inlet kg T1 20o C s 0.9456 kJ 1 kg K x1 1.0 kJ Compressor exit h2 s 278.23 kg P2 s P2 900 kPa o kJ T2 s 43.79 C s2 s s1 0.9456 kg K kJ h 101.61 3 Condenser exit kg P3 900 kPa kJ s3 0.3738 kg K x3 0.0 x 0.358 Throttle exit 4 kJ T4 T1 20o C s4 0.4053 kg K h4 h3 State1 State 3 State 2 State 4 41 COPR QL m(h1 h4 ) h1 h4 Wnet , in m(h2 h1 ) h2 h1 kJ (238.41 101.61) kg kJ (278.23 238.41) kg 3.44 The tons of refrigeration, often called the cooling load or refrigeration effect, are QL m(h1 h4 ) kg kJ 1Ton (238.41 101.61) min kg 211 kJ min 1.94 Ton 3 COPR , Carnot TL TH TL (20 273) K (43.79 ( 20)) K 3.97 42 Another measure of the effectiveness of the refrigeration cycle is how much input power to the compressor, in horsepower, is required for each ton of cooling. The unit conversion is 4.715 hp per ton of cooling. Wnet , in QL 4.715 COPR 4.715 hp 3.44 Ton hp 1.37 Ton 43 Actual Vapor-Compression Refrigeration Cycle An actual vapor-compression refrigeration cycle differs from the ideal one in several ways, owing mostly to the irreversibilities that occur in various components. Two common sources of irreversibilities are fluid friction (causes pressure drops) and heat transfer to or from the surroundings. The T-s diagram of an actual vapor-compression refrigeration cycle is shown in the Fig. In the ideal cycle, the refrigerant leaves the evaporator and enters the compressor as saturated vapor. In practice, however, it may not be possible to control the state of the refrigerant so precisely. Instead, it is easier to design the system so that the refrigerant is slightly superheated at the compressor inlet. This slight overdesign ensures that the refrigerant is completely vaporized when it enters the compressor. Also, the line connecting the evaporator to the compressor is usually very long; thus the pressure drop caused by fluid friction and heat transfer from the surroundings to the refrigerant can be very significant. The result of superheating, heat gain in the connecting line, and pressure drops in the evaporator and the connecting line is an increase in the specific volume, thus an increase in the power input requirements to the compressor since steady-flow work is proportional to the specific volume. Actual Vapor-Compression Refrigeration Cycle The compression process in the ideal cycle is internally reversible and adiabatic, and thus isentropic. The actual compression process, however, involves frictional effects, which increase the entropy, and heat transfer, which may increase or decrease the entropy, depending on the direction. Therefore, the entropy of the refrigerant may increase (process 1-2) or decrease (process 1-2’) during an actual compression process, depending on which effects dominate. The compression process 1-2’ may be even more desirable than the isentropic compression process since the specific volume of the refrigerant and thus the work input requirement are smaller in this case. Therefore, the refrigerant should be cooled during the compression process whenever it is practical and economical to do so. Actual Vapor-Compression Refrigeration Cycle In the ideal case, the refrigerant is assumed to leave the condenser as saturated liquid at the compressor exit pressure. In reality, however, it is unavoidable to have some pressure drop in the condenser as well as in the lines connecting the condenser to the compressor and to the throttling valve. Also, it is not easy to execute the condensation process with such precision that the refrigerant is a saturated liquid at the end, and it is undesirable to route the refrigerant to the throttling valve before the refrigerant is completely condensed. Therefore, the refrigerant is subcooled somewhat before it enters the throttling valve. We do not mind this at all, however, since the refrigerant in this case enters the evaporator with a lower enthalpy and thus can absorb more heat from the refrigerated space. The throttling valve and the evaporator are usually located very close to each other, so the pressure drop in the connecting line is small Actual Vapor-Compression Refrigeration Cycle 47 Vapour compression method of refrigeration The most frequently used refrigeration cycle is the vapor-compression refrigeration cycle, which involves four main components: a compressor, a condenser, an expansion valve, and an evaporator, as shown in Fig. Schematic of a basic vapour compression refrigeration system Vapour compression method of refrigeration Figure shows the basic components of a vapour compression refrigeration system. As shown in the figure the basic system consists of an evaporator, compressor, condenser and an expansion valve. The refrigeration effect is obtained in the cold region as heat is extracted by the vaporization of refrigerant in the evaporator. The refrigerant vapour from the evaporator is compressed in the compressor to a high pressure at which its saturation temperature is greater than the ambient or any other heat sink. Hence when the high pressure, high temperature refrigerant flows through the condenser, condensation of the vapour into liquid takes place by heat rejection to the heat sink. To complete the cycle, the high pressure liquid is made to flow through an expansion valve. In the expansion valve the pressure and temperature of the refrigerant decrease. This low pressure and low temperature refrigerant vapour evaporates in the evaporator taking heat from the cold region. It should be observed that the system operates on a closed cycle. The system requires input in the form of mechanical work. It extracts heat from a cold space and rejects heat to a high temperature heat sink. Fig. Vapour compression method of refrigeration A vapour compression refrigeration system can also be used as a heat pump, in which the useful output is the high temperature heat rejected at the condenser. Alternatively, a refrigeration system can be used for providing cooling in summer and heating in winter. Such systems have been built and are available now. HEAT PUMP SYSTEMS Heat pumps are generally more expensive to purchase and install than other heating systems, but they save money in the long run in some areas because they lower the heating bills. Despite their relatively higher initial costs, the popularity of heat pumps is increasing. About one-third of all single-family homes built in the United States in the last decade are heated by heat pumps. The most common energy source for heat pumps is atmospheric air (air-to-air systems), although water and soil are also used. The major problem with air-source systems is frosting, which occurs in humid climates when the temperature falls below 2 to 5°C. The frost accumulation on the evaporator coils is highly undesirable since it seriously disrupts heat transfer. The coils can be defrosted, however, by reversing the heat pump cycle (running it as an air conditioner). This results in a reduction in the efficiency of the system. Water-source systems usually use well water from depths of up to 80m in the temperature range of 5 to 18°C, and they do not have a frosting problem. They typically have higher COPs but are more complex and require easy access to a large body of water such as underground water. Ground source systems are also rather involved since they require long tubing placed deep in the ground where the soil temperature is relatively constant. The COP of heat pumps usually ranges between 1.5 and 4, depending on the particular system used and the temperature of the source. A new class of recently developed heat pumps that use variable-speed electric motor drives are at least twice as energy efficient as their predecessors. HEAT PUMP SYSTEMS Both the capacity and the efficiency of a heat pump fall significantly at low temperatures. Therefore, most air-source heat pumps require a supplementary heating system such as electric resistance heaters or an oil or gas furnace. Since water and soil temperatures do not fluctuate much, supplementary heating may not be required for water-source or ground-source systems. However, the heat pump system must be large enough to meet the maximum heating load. Heat pumps and air conditioners have the same mechanical components. Therefore, it is not economical to have two separate systems to meet the heating and cooling requirements of a building. One system can be used as a heat pump in winter and an air conditioner in summer. This is accomplished by adding a reversing valve to the cycle, as shown in Fig. in the next slide. A sa result of this modification, the condenser of the heat pump (located indoors) functions as the evaporator of the air conditioner in summer. Also, the evaporator of the heat pump (located outdoors) serves as the condenser of the air conditioner. This feature increases the competitiveness of the heat pump. Such dual-purpose units are commonly used in motels. Heat pumps are most competitive in areas that have a large cooling load during the cooling season and a relatively small heating load during the heating season, such as in the southern parts of the United States. In these areas, the heat pump can meet the entire cooling and heating needs of residential or commercial buildings. The heat pump is least competitive in areas where the heating load is very large and the cooling load is small, such as in the northern parts of the United States. Heat Pump Systems 53 Vapour Absorption Refrigeration Systems Another form of refrigeration that becomes economically attractive when there is a source of inexpensive thermal energy at a temperature of 100 to 200°C is absorption refrigeration. Some examples of inexpensive thermal energy sources include geothermal energy, solar energy, and waste heat from cogeneration or process steam plants, and even natural gas when it is available at a relatively low price. As the name implies, absorption refrigeration systems involve the absorption of a refrigerant by a transport medium. The most widely used absorption refrigeration system is the ammonia–water system, where ammonia (NH3) serves as the refrigerant and water (H2O) as the transport medium. Other absorption refrigeration systems include water–lithium bromide and water– lithium chloride systems, where water serves as the refrigerant. The latter two systems are limited to applications such as air-conditioning where the minimum temperature is above the freezing point of water. Vapour Absorption Refrigeration Systems To understand the basic principles involved in absorption refrigeration, we examine the NH3–H2O system shown in the Fig. The ammonia–water refrigeration machine was patented by the Frenchman Ferdinand Carre in 1859. Within a few years, the machines based on this principle were being built in the United States primarily to make ice and store food. You will immediately notice from the figure that this system looks very much like the vapor-compression system, except that the compressor has been replaced by a complex absorption mechanism consisting of an absorber, a pump, a generator, a regenerator, a valve, and a rectifier. Once the pressure of NH3 is raised by the components in the box (this is the only thing they are set up to do), it is cooled and condensed in the condenser by rejecting heat to the surroundings, is throttled to the evaporator pressure, and absorbs heat from the refrigerated space as it flows through the evaporator. So, there is nothing new there. Here is what happens in the box: Ammonia vapor leaves the evaporator and enters the absorber, where it dissolves and reacts with water to form NH3H2O . This is an exothermic reaction; thus heat is released during this process. The amount of NH3 that can be dissolved in H2O is inversely proportional to the temperature. Therefore, it is necessary to cool the absorber to maintain its temperature as low as possible, hence to maximize the amount of NH3 dissolved in water. The liquid NH3 + H2O solution, which is rich in NH3, is then pumped to the generator. Heat is transferred to the solution from a source to vaporize some of the solution. The vapor, which is rich in NH3, passes through a rectifier, which separates the water and returns it to the generator. The high-pressure pure NH3 vapor then continues its journey through the rest of the cycle. Absorption Refrigeration Systems Another form of refrigeration that becomes economically attractive when there is a source of inexpensive heat energy at a temperature of 100 to 200oC is absorption refrigeration, where the refrigerant is absorbed by a transport medium and compressed in liquid form. The most widely used absorption refrigeration system is the ammonia-water system, where ammonia serves as the refrigerant and water as the transport medium. The work input to the pump is usually very small, and the COP of absorption refrigeration systems is defined as COPR Ammonia absorption refrigeration cycle Desired output Cooling effect QL Q L Required input Work input Qgen Wpump ,in Qgen 56 Vapour Absorption Refrigeration Systems The hot NH3 + H2O solution, which is weak in NH3, then passes through a regenerator, where it transfers some heat to the rich solution leaving the pump, and is throttled to the absorber pressure. Compared with vapor-compression systems, absorption refrigeration systems have one major advantage: A liquid is compressed instead of a vapor. The steady-flow work is proportional to the specific volume, and thus the work input for absorption refrigeration systems is very small (on the order of one percent of the heat supplied to the generator) and often neglected in the cycle analysis. The operation of these systems is based on heat transfer from an external source. Therefore, absorption refrigeration systems are often classified as heat-driven systems. The absorption refrigeration systems are much more expensive than the vapor-compression refrigeration systems. They are more complex and occupy more space, they are much less efficient thus requiring much larger cooling towers to reject the waste heat, and they are more difficult to service since they are less common. Therefore, absorption refrigeration systems should be considered only when the unit cost of thermal energy is low and is projected to remain low relative to electricity. Absorption refrigeration systems are primarily used in large commercial and industrial installations. Multistage refrigeration systems Multistage refrigeration systems are used where ultralow temperatures are required but cannot be obtained economical through the use of a single-stage system. The reason for this is the compression ratios are too high to attain the temperatures required to evaporate and condense the vapour. The following two general types of systems are presently in use: • Cascade • Compound Other Refrigeration Cycles Cascade refrigeration systems Very low temperatures can be achieved by operating two or more vapor-compression systems in series, called cascading. The COP of a refrigeration system also increases as a result of cascading. The cascade system has two separate refrigerant systems interconnected in such away that the evaporator from the first unit cools the condenser of the second unit. This allows one of the units to be operated at a lower temperature and pressure than would otherwise be possible with the same type and size of single-stage system. It also allows two different refrigerants to be used, and it can produce temperatures as low as −50°F 59 The compound system The compound system uses two or more compressors connected in series in the same refrigeration system. In this type of system the first stage compressor is the largest and for each succeeding stage the compressor gets smaller. This is because as the refrigerant passes through each compressor, it becomes a denser vapour. A two-stage compound system can attain a temperature of approximately –80°F. A three-stage system (Figure) can attain a temperature of –135°F efficiently. Compressor 1 pumps vapour into the intercooler and then into the intake of compressor 2. This operation is repeated between the second and third stages. In the third stage, the refrigerant vapour is further cooled and travels to the evaporator for specific cooling use. Compound refrigeration system (three-stage) Multipurpose refrigeration systems A refrigerator with a single compressor can provide refrigeration at several temperatures by throttling the refrigerant in stages. 62 Liquefaction of gases Another way of improving the performance of a vapor-compression refrigeration system is by using multistage compression with regenerative cooling. The vaporcompression refrigeration cycle can also be used to liquefy gases after some modifications. 63 Gas Refrigeration Systems The power cycles can be used as refrigeration cycles by simply reversing them. Of these, the reversed Brayton cycle, which is also known as the gas refrigeration cycle, is used to cool aircraft and to obtain very low (cryogenic) temperatures after it is modified with regeneration. The work output of the turbine can be used to reduce the work input requirements to the compressor. Thus, the COP of a gas refrigeration cycle is COPR qL qL wnet , in wcomp , in wturb , out 64 Methods of reproducing refrigeration Evaporative cooling As the name indicates, evaporative cooling is the process of reducing the temperature of a system by evaporation of liquid (i.e. water). Human beings perspire and dissipate their metabolic heat by evaporative cooling if the ambient temperature is more than skin temperature. Animals such as the hippopotamus and buffalo coat themselves with mud for evaporative cooling. Evaporative cooling has been used for centuries to obtain cold water in summer by storing the water in earthen pots. The water permeates through the pores of earthen vessel to its outer surface where it evaporates to the surrounding, absorbing its latent heat in part from the vessel, which cools the water. Evaporative cooling is based on the thermodynamics of evaporation of water i.e. the change of liquid phase of Water into water vapor. This change phase requires energy which is called the latent heat of evaporation. This is the energy required to change a substance from liquid phase to gaseous one without temperature. When evaporation occurs naturally it called passive evaporation and evaporation has to controlled by means of some mechanical device, the system is called hybrid evaporative system. Thermoelectric Refrigeration Thermoelectric refrigeration is a novel method of producing low temperatures and is based on the reverse Seebeck effect. The Figure shows the illustration of Seebeck and Peltier effects. As shown, in Seebeck effect an EMF, E is produced when the junctions of two dissimilar conductors are maintained at two different temperatures T1 and T2. This principle is used for measuring temperatures using thermocouples. Experimental studies show that Seebeck effect is reversible. The electromotive force produced is given by: E =α(T1 −T2 ) where α is the thermoelectroic power or Seebeck coefficient. For a constant cold junction temperature (T2), α= dE/dT Illustration of Seebeck and Peltier effects Thermoelectric Refrigeration If a closed circuit is formed by the conductors, then an electrical current, I flows due to the emf and this would result in irreversible generation of heat (qir= I2R) due to the finite resistance R of the conductors. This effect is known as Joulean Effect. Due to different temperatures T1 and T2 (T1>T2), there will be heat transfer by conduction also. This is also irreversible and is called as conduction effect. The amount of heat transfer depends on the overall thermal conductance of the circuit. When a battery is added in between the two conductors A and B whose junctions are initially at same temperature, and a current is made to flow through the circuit, the junction temperatures will change, one junction becoming hot (T1) and the other becoming cold (T2). This effect is known as Peltier effect. Refrigeration effect is obtained at the cold junction and heat is rejected to the surroundings at the hot junction. This is the basis for thermoelectric refrigeration systems. The position of hot and cold junctions can be reversed by reversing the, direction of current flow. The heat transfer rate at each junction is given by: Q= φI where φ is the Peltier coefficient in volts and I is the current in amperes. Thermoelectric Refrigeration When current is passed through a conductor in which there is an initial uniform temperature gradient, then it is observed that the temperature distribution gets distorted as heat transfer takes place. This effect is known as Thomson effect. POSITIVE THOMSON EFFECT In metals such as zinc and copper, which have a hotter end at a higher potential and a cooler end at a lower potential, when current moves from the hotter end to the colder end, it is moving from a high to a low potential, so there is an evolution of heat. This is called the positive Thomson effect. NEGATIVE THOMSON EFFECT In metals such as cobalt, nickel, and iron, which have a cooler end at a higher potential and a hotter end at a lower potential, when current moves from the hotter end to the colder end, it is moving from a low to a high potential, there is an absorption of heat. This is called the negative Thomson effect. Thermoelectric Refrigeration The Joule-Thomson effect : The reduction of temperature experienced by a gas when throttled from a high pressure to a lower one. This is known as the Joule-Thompson effect. This expansion can be carried out: - Without external work being applied to a valve or passage through an orifice. The process is accompanied by a small temperature drop. - With external work being applied to an expansion machine. In this case expansion corresponds to a considerable drop in the temperature of the gas being expansion. The Joule-Thomson effect (throttling effect is observed as refrigerant flows across the thermostatic expansion valve. A similar effect is noticed as high pressure LPG gas flows out a cylinder. The Joule–Thomson effect is widely used in cryogenic engineering and in the liquefaction of gases. RANQUE TUBE METHOD (RANQUE EFFECT) The vortex tube, also known as the Ranque-Hilsch vortex tube, is a device that separates a compressed gas into hot and cold streams. It has no moving parts. Pressurized gas is injected tangentially into a swirl chamber and accelerates to a high rate of rotation. Due to the conical nozzle at the end of the tube, only the outer shell of the compressed gas is allowed to escape at that end as hot air. The remainder of the gas is forced to return in an inner vortex of reduced diameter within the outer vortex as cold air. There are different explanations for the effect and there is debate on which explanation is best or correct. One simple explanation is that the outer air is under higher pressure than the inner air (because of centrifugal force). Therefore the temperature of the outer air is higher than that of the inner air. Ranque-Hilsch Effect Tube ("vortex tube") Expandable refrigeration An expendable refrigeration system is for used in trucks, railroad cars, and shipping containers that transport perishable items. The three types of refrigerants presently being used in an expendable system are liquid nitrogen, carbon dioxide, and liquid helium. The evaporator system and the spray system are two types of expendable systems commonly used in the Navy. Expandable evaporator refrigeration system In the expendable evaporator system, liquid refrigerant is stored in large metal insulated cylinders. These cylinders are normally located in the front of the cargo vehicle. Each cylinder is equipped with a temperature control to provide a temperature range of –20°F to 60°F. The temperature control is connected to a temperature sensor. As the temperature rises, the switch operating the control valve opens and liquid refrigerant flows into the evaporator. The evaporator can be blower coils, plates, or eutectic plates. As it passes through the evaporator, the refrigerant vaporizes. The vapor is pushed through the evaporator by the pressure difference between the cylinder and the vent. When the selected temperature is attained, the refrigerant valve closes. The vapor that has been used is then discharged from the evaporator at about the same temperature as the air in the cargo vehicle. With this system, the refrigerant does not mix with air in the vehicle space. An example of the expendable evaporator system is shown in the Figure. This example shows two nitrogen cylinders located inside a truck body connected by a manifold to regulators and to temperature control solenoid valves. The vaporizing liquid nitrogen flows into the vaporizers or cold plates to refrigerate the true box. Expandable evaporator refrigeration system Spray Systems In the expendable refrigerant spray system, liquid nitrogen or carbon dioxide is sprayed directly into the vehicle space that is to be cooled. This system uses liquid containers, a control box, a fill box, spray headers, emergency switches, and safety vents. The fill box is normally located on the front of the vehicle. It contains the valves, gauges, and connections that allow the liquid containers to be filled. The liquid containers are insulated cylinders similar to thermos bottles. The control box contains the valves, gauges, and thermostats that are necessary for safe release of the liquid to the spray headers. Once the liquid is received at the spray headers, the nozzles spray it into the vehicle. The remaining two components are primarily safety devices. These emergency interlock switches are attached to each door. That means, whenever a door is opened, the system shuts down. The safety vent is a small trapdoor that vents air directly to the atmosphere whenever the air inside the truck box exceeds atmospheric pressure. A benefit of this system is that liquid nitrogen or carbon dioxide replaces the oxygen inside the space being refrigerated. Therefore when fruits, vegetables, meats, and fish are being refrigerated, they are also preserved by the inert atmosphere. The Fig. illustrates the spray system. Liquid nitrogen (dark red) supplied from a cylinder inside the refrigerated space, kept under pressure (200 psi). Dark blue indicates low pressure liquid refrigerant. Primary and Secondary refrigerant Refrigerant It is a substance in the vapour state which used as a working fluid in refrigerators. It is also a cooling agent refrigeration system. Important practical issues such as the system design, size, initial and operating costs, safety, reliability, and serviceability etc. depend very much on the type of refrigerant selected for a given application. Due to several environmental issues such as ozone layer depletion and global warming and their relation to the various refrigerants used, the selection of suitable refrigerant has become one of the most important issues in recent times. Classification of refrigerants • Primary refrigerant • Secondary refrigerant Primary refrigerants cools substance or space directly by absorbing heat. It is also known as direct expansion system. They are used directly as working fluids, for example in vapour compression and vapour absorption refrigeration systems. When used in compression or absorption systems, these fluids provide refrigeration by undergoing a phase change process in the evaporator. Example are Ammonia, Freon, SO2, CO2 etc Ammonia (NH3) • Used for commercial purposes mainly in cold stores and ice plants • The boiling temperature of NH3 is -330oC • It is colourless gas with a pungent smell • Has good thermodynamics properties • It is neutral to all metal, highly soluble in oil • Its solubility increase with increasing pressure and decreasing temperature • Used free small and medium refrigerating capacities • Volatile and toxic under ambient temperature Sulphur Dioxide SO2 • Previously used in household refrigerator • Toxic, non-explosive and non-flammable and non-corrosive • Irritant to the human body • Non-mixable with oil • Has pungent odour and low latent heat value Carbon Dioxide • • • • • Colourless gas at ordinary temperatures It has a slight odour and acid taste It has a boiling point of 5oF (15 oC) It is harmless to breathe except in large concentrations Carbon dioxide is used aboard ship and industrial installation • It is not used in household application • The main advantage using carbon dioxide as a refrigerant is that small compressors can be used. • It is inefficient, compared other refrigerant Freon group • They are fluorocarbons of methane and ethane series • They contain one or more of these halogens (chlorine, bromine, fluorine) • Non-toxic, non-flammable, non-corrosive, non-explosive, nonirritant to the human body and eyes • Odourless and colourless • Will not react with food product stored in the refrigerated space. • Will not react with lubrication oil • Has excellent thermodynamics properties • Only disadvantage is the ozone layer is damaged Freon 11(Trichloro monofluro methane (CCl3F) • Has boiling point of 74.9oF (23.8oC) • Used in centrifugal compressors for industrial and commercial air conditioning plants in a larger level factories and theatres • It is also used for industrial process water and brine cooling • Its low viscosity and freezing point have also led to its use as low temperature brine. Freon 12 (Dichloro-difluro methane- CCl2F2) • Most used in domestic and commercial refrigeration system ( in ice cream, display cabinet, deep freezers, food locker plants, water coolers, window air-conditioning unit etc) • It has a boiling point of -21.6oF (-29.8oC) • Most widely used and known of the Freon refrigerant • It is widely used colourless gas with mild odour • Heavier than air • Does not dissolve in water • Refrigeration effect per unit volume of ammonia is 1.5times that of Freon – 12 • It does not react with ferrous metals, aluminium • It attack copper, copper alloys, zinc, and bronze Freon 13 (Mono chloro-trifluoromethane(CClF3) • It has a boiling point of -41.4oF (-40.8oC) • They are used in small refrigeration plants • Used to obtain moderately low temperature (500oC) • Generally used in cascade with Freon 12, Freon 22 or 522 Look for the characteristics of Freon 22, Freon 113, Freon 114, Freon 500, Freon 503 Secondary refrigerant Secondary refrigerants are those liquids, which are used for transporting thermal energy from one location to other. Secondary refrigerants are also known under the name brines or antifreezes Antifreezes or brines are used when refrigeration is required at sub-zero temperatures. Unlike primary refrigerants, the secondary refrigerants do not undergo phase change as they transport energy from one location to other. An important property of a secondary refrigerant is its freezing point. Generally, the freezing point of a brine will be lower than the freezing point of its constituents. The temperature at which freezing of a brine takes place its depends on its concentration. The concentration at which a lowest temperature can be reached without solidification is called as eutectic point. The commonly used secondary refrigerants are the solutions of water and ethylene glycol, propylene glycol or calcium chloride. These solutions are known under the general name of brines. Calcium Chloride (CaCl2) • It is used only in commercial refrigeration plants. • Simple used as a carrying medium for refrigeration • Used in large installations were there is danger of leakage • Also used where the temperature fluctuates in the space to be refrigerated • They cooled down by direct expansion of the refrigerant. It is then pumped through the material or space to be cooled. Ethyl Chloride (C5H5Cl) • It not is commonly used in domestic refrigeration units • It is colourless liquid or gas with pungent ethereal odour and sweetish taste • It is neutral towards all metals • It soften all rubber compounds and gasket material Refrigerant selection criteria Selection of refrigerant for a particular application is based on the following requirements: i. Thermodynamic and thermo-physical properties ii. Environmental and safety properties, and iii. Economics Thermodynamic and thermo-physical properties a) Suction pressure: At a given evaporator temperature, the saturation pressure should be above atmospheric for prevention of air or moisture ingress into the system and ease of leak detection. Higher suction pressure is better as it leads to smaller compressor displacement b) Discharge pressure: At a given condenser temperature, the discharge pressure should be as small as possible to allow light-weight construction of compressor, condenser etc. c) Pressure ratio: Should be as small as possible for high volumetric efficiency and low power consumption d) Latent heat of vaporization: Should be as large as possible so that the required mass flow rate per unit cooling capacity will be small In addition to the above properties; the following properties are also important e) Isentropic index of compression: Should be as small as possible so that the temperature rise during compression will be small f) Liquid specific heat: Should be small so that degree of subcooling will be large leading to smaller amount of flash gas at evaporator inlet g) Vapour specific heat: Should be large so that the degree of superheating will be small h) Thermal conductivity: Thermal conductivity in both liquid as well as vapour phase should be high for higher heat transfer coefficients i) Viscosity: Viscosity should be small in both liquid and vapour phases for smaller frictional pressure drops Environmental and safety properties a) Ozone Depletion Potential (ODP): According to the Montreal protocol, the ODP of refrigerants should be zero, i.e., they should be non-ozone depleting substances. Refrigerants having non-zero ODP have either already been phasedout (e.g. R11, R12) or will be phased-out in near-future(e.g. R22). Since ODP depends mainly on the presence of chlorine or bromine in the molecules, refrigerants having either chlorine (i.e., CFCs and HCFCs) or bromine cannot be used under the new regulations b) Global Warming Potential (GWP): Refrigerants should have as low a GWP value as possible to minimize the problem of global warming. Refrigerants with zero ODP but a high value of GWP (e.g. R134a) are likely to be regulated in future. c) Total Equivalent Warming Index (TEWI): The factor TEWI considers both direct (due to release into atmosphere) and indirect (through energy consumption) contributions of refrigerants to global warming. Naturally, refrigerants with as a low a value of TEWI are preferable from global warming point of view. d) Toxicity: Ideally, refrigerants used in a refrigeration system should be non-toxic. However, all fluids other than air can be called as toxic as they will cause suffocation when their concentration is large enough. Thus toxicity is a relative term, which becomes meaningful only when the degree of concentration and time of exposure required to produce harmful effects are specified. Some fluids are toxic even in small concentrations. Some fluids are mildly toxic, i.e., they are dangerous only when the concentration is large and duration of exposure is long. Some refrigerants such as CFCs and HCFCs are non-toxic when mixed with air in normal condition. However, when they come in contact with an open flame or an electrical heating element, they decompose forming highly toxic elements (e.g. phosgene- COCl2). In general the degree of hazard depends on: Other important properties e) Flammability: The refrigerants should preferably be non-flammable and non-explosive. For flammable refrigerants special precautions should be taken to avoid accidents. f) Chemical stability: The refrigerants should be chemically stable as long as they are inside the refrigeration system. g) Compatibility with common materials of construction (both metals and nonmetals) h) Miscibility with lubricating oils: Oil separators have to be used if the refrigerant is not miscible with lubricating oil (e.g. ammonia). Refrigerants that are completely miscible with oils are easier to handle (e.g. R12). However, for refrigerants with limited solubility (e.g. R 22) special precautions should be taken while designing the system to ensure oil return to the compressor j) Ease of leak detection: In the event of leakage of refrigerant from the system, it should be easy to detect the leaks. Economic properties The refrigerant used should preferably be inexpensive and easily available. Other classification Azeotropic mixtures Although it contains two or more refrigerants, at a certain pressure an azeotropic mixture evaporates and condenses at a constant temperature. Because of this, azeotropic mixtures behave like pure refrigerants in all practical aspects. Azeotropic mixtures are designated by 500 series, whereas zeotropic refrigerants (e.g. non-azeotropic mixtures) are designated by 400 series. Azeotropic mixtures: R 500: Mixture of R 12 (73.8 %) and R 152a (26.2%) R 502: Mixture of R 22 (48.8 %) and R 115 (51.2%) R503: Mixture of R 23 (40.1 %) and R 13 (59.9%) R507A: Mixture of R 125 (50%) and R 143a (50%) Non-azeotropic/Zeotropic mixtures Zeotropic mixtures have a gliding evaporation and condensing temperature. When evaporating, the most volatile component will boil off first and the least volatile component will boil off last. The opposite happens when gas condenses into liquid. Zeotropic mixtures: R404A : Mixture of R 125 (44%), R 143a (52%) and R 134a (4%) R407A : Mixture of R 32 (20%), R 125 (40%) and R 134a (40%) R407B : Mixture of R 32 (10%), R 125 (70%) and R 134a (20%) R410A : Mixture of R 32 (50%) and R 125 (50%) The Pressure - Enthalpy Chart Latent Heat of Fusion - The quantity of heat (Btu/lb) required to change 1 lb. of material from the solid phase into the liquid phase. Latent Heat of Vaporization - The quantity of heat (Btu/lb) required to change 1 lb. of material from the liquid phase into the vapor phase. Sensible Heat - Heat that is absorbed/rejected by a material, resulting in a change of temperature. Latent Heat - Heat that is absorbed/rejected by a material resulting in a change of physical state (occurring at constant temperature). Saturation Temperature - That temperature at which a liquid starts to boil (or vapor starts to condense). The saturation temperature (boiling temperature) is constant at a given pressure,* and increases as the pressure increases. A liquid cannot be raised above its saturation temperature. Whenever the refrigerant is present in two states (liquid and vapor) the refrigerant mixture will be at the saturation temperature. Superheat - At a given pressure, the difference between a vapor’s temperature and its saturation temperature. Subcooling - At a given pressure, the difference between a liquid’s temperature and its saturation temperature. The Pressure - Enthalpy Chart The Pressure - Enthalpy Chart Pressure - The vertical axis of the chart, in psia (see pink line). To obtain gauge pressure, subtract atmospheric pressure. Enthalpy - The horizontal axis of the chart shows the heat content of the refrigerant in Btu/lb. Temperature- Constant temperature lines generally run in a vertical direction in the superheated vapor & subcooled liquid portion of the chart. In the saturated bubble, the constant temperature line is along the horizontal, illustrating that the saturation temperature is constant at a given pressure (see black line). Specific Volume - Constant volume lines extend from the red line saturated vapor line out into the superheated vapor-portion of the chart at a slight angle from the horizontal axis. Specific volume is expressed in cu.ft/lb. (see orange line). Entropy - Entropy is the mathematical relationship between heat and temperature, and relates to the availability of energy. These lines extend at an angle from the saturated vapor line. Their presence on the chart is relevant in that vapor compression (in the ideal cycle) occurs at constant entropy (see dark blue line). Quality - Lines of constant quality appear vertically, and only within the saturation bubble. The refrigerant within the bubble is a mixture of liquid and vapor at saturation, and the quality is the percentage of the mixture which is in the vapor state (see green line). The Ideal cycle If the operating temperatures and pressures are known, the refrigeration system can be plotted on the P-H diagram. Let’s assume our system is operating at a -20ºF evaporator and 100ºF condensing. Data Points and System Design Calculations Refrigeration Effect (RE): This is the total heat transfer, in Btu/lb, from the refrigerated space to the refrigerant. H1 minus H4 (H1 is the enthalpy of the refrigerant at point #1 in Fig. 3, and so forth). Heat of Compression (HOC):This is the amount of heat added to the refrigerant from the compression process (represented by H2 minus H1 on the chart). Heat of Rejection (HOR): This is the amount of heat that has to be rejected at the condenser. The heat transferred to the refrigerant from the refrigerated space (RE), and the heat transferred to the refrigerant during compression (HOC). It is this value, plus some safety factor, from which the condenser selection is made (represented by H2 minus H3 on the chart Data Points and System Design Calculations Note: For the purpose of this discussion, H1 will be considered the point where the evaporation line intersects with the saturated vapor line. In the real world, the location of H1 would be to the right of the saturated vapor line, reflecting the superheated vapor at the evaporator outlet. With an expansion valve maintaining a typical amount of superheat (in the 4° - 6° range for low temperature applications), the heat transferred to the vapor is minimal (less than 1 Btu/lb). Let’s look at a few typical system scenarios, plot them on the P-H diagram, and then compare the performance measurements. Typical Cycle #1 (Fig. 4): It is neither realistic nor safe to have a saturated vapor at the compressor inlet. Because liquid cannot be present with superheated vapor, some amount of superheat at the compressor inlet becomes the margin necessary to insure the safety of the compressor This is the result of an expansion valve set to maintain some amount of superheat, plus the temperature increase the refrigerant vapor experiences in the suction line. The suction superheat will insure that the compressor is protected from liquid flooding. The cost of this protection comes in the form of a larger compressor volume requirement. Typical Cycle #1A (Fig. 5): Using an open drive compressor, with a 20ºF vapor temperature at the compressor inlet, we see the benefit of subcooling the liquid to 50ºF. Note the change in refrigerant quality at the TEV outlet. Instead of 65% liquid, we now have 80% liquid. Because the difference between liquid temperature and evaporator temperature has been reduced, there is less refrigerant flashing during the expansion process. The benefit realized resulting from subcooling will be offset by the higher suction vapor temperatures, and the volume requirement penalty they impose. Typical Cycle #2 (Fig. 6): The advantage of using a hermetic compressor is the elimination of either belts or drive motor couplings which require precise alignment, and crankshaft seals. The disadvantage is that there is now an electric motor in the refrigerant circuit (at least on suction vapor cooled hermetic compressors). In addition to the guaranteed system contamination problem when a hermetic motor burns, you have the heat from the motor being transferred to the refrigerant vapor. An approximate 80ºF temperature increase can be expected between the vapor entering the compressor service valve, and the vapor entering the compressor cylinders. This brings the suction vapor temperature up to 100ºF, with a corresponding increase in discharge temperature…now approaching 300ºF. This is the upper limit at which most compressor manufacturers agree shouldn’t be exceeded. The higher suction vapor temperature also results in a higher HOC, which raises the horsepower requirement. Dirty condenser Filthy condenser and TEV not adjusted Typical Cycle #4 (Fig. 8): Not only is the condenser filthy, but the TEV’s were never adjusted. They are operating at abnormally high superheats, which in effect have reduced the size of the evaporators. As a result, the discharge air temperature in the glass door frozen food display cases is too high, causing the frozen juice to melt. Deviation of actual cycle from ideal cycle Irreversibilities that occur in various components. Two common sources of irreversibilities are fluid friction (causes pressure drops) in the tubes, valves and other accessories and heat transfer to or from the surroundings. The compression process occurs at a constant entropy ONLY in the ideal cycle. In the real world, entropy will increase during the compression process, resulting in even higher discharge temperatures and HOC values Compressors Function of the compressor It is a pump, like the heart in the circulation system of the human body. It is considered as the heart of the refrigeration system. In the refrigeration system, it is responsible for pumping vapour i.e. it is also referred to as vapour pump. The compressor lifts/increase the pressure of the ref system from suction pressure level (low pressure) to discharge level. All compressors are powered or driven by an electric motor. Compression ratio (CR) The compression ratio is an engineering expression of the high side absolute pressure divided by the low side absolute pressure. It is expressed in absolute pressures. Compression ratio (CR) = 𝑎𝑏𝑠𝑜𝑙𝑢𝑡𝑒 𝑑𝑖𝑠𝑐ℎ𝑎𝑟𝑔𝑒 𝑝𝑟𝑒𝑠𝑠𝑢𝑟𝑒 𝑎𝑏𝑠𝑜𝑙𝑢𝑡𝑒 𝑠𝑢𝑐𝑡𝑖𝑜𝑛 𝑝𝑟𝑒𝑠𝑠𝑢𝑟𝑒 NB: The difference between the discharge pressure and the suction pressure is referred to as the lifts pressure Performance of a compressor The performance of a compressor is influenced by numerous parameters including the following: • compressor speed, • suction pressure and temperature, • discharge pressure and temperature, and • type of refrigerant and its flow rate. Classification of compressors Compressors used in refrigeration systems can be classified in several ways: Based on working principles or arrangement of compressor motor or external drive. The method of compression may be reciprocating, rotary and centrifugal Based on working principles • Positive displacement type • Roto-dynamic type Positive displacement compressors These compressors use the shaft work to increase the refrigerant pressure by reducing the compression volume in the chamber. The compressors of this group are reciprocating, vane (rotary), and screw (helical rotary) compressors. Positive displacement compressors In positive displacement type of compressors, compression is achieved by trapping a refrigerant vapour into an enclosed space and then reducing its volume. Since a fixed amount of refrigerant is trapped each time, its pressure rises as its volume is reduced. When the pressure rises to a level that is slightly higher than the condensing pressure, then it is expelled from the enclosed space and a fresh charge of low-pressure refrigerant is drawn in and the cycle continues. Since the flow of refrigerant to the compressor is not steady, the positive displacement type compressor is a pulsating flow device. However, since the operating speeds are normally very high the flow appears to be almost steady on macroscopic time scale. Since the flow is pulsating on a microscopic time scale, positive displacement type compressors are prone to high wear, vibration and noise level. Positive displacement compressors Depending upon the construction, positive displacement type compressors used in refrigeration and air conditioning can be classified into: • Reciprocating, • Vane (rotary), and • Screw (helical rotary) compressors Reciprocating compressors Reciprocating compressor is the workhorse of the refrigeration and air conditioning industry. It is the most widely used compressor with cooling capacities ranging from a few Watts to hundreds of kilowatts. Modern day reciprocating compressors are high speed (≈ 3000 to 3600 rpm), single acting, single or multi-cylinder (up to 16 cylinders) type. Reciprocating compressors Schematic diagram of a reciprocating compressor Reciprocating compressors • It consists of a piston moving back and forth in a cylinder, with suction and discharge valves to achieve suction and compression of refrigerant vapour. • The construction and working principles are similar two stroke engine, where suction and compression are completed in revolution of a crank. • The suction side of the compressor is connected to the exit of the evaporator, while the discharge side of the compressor is connected to the condenser inlet. • The suction (inlet) and the discharge (outlet) valves open and close due to pressure differences between the cylinder and inlet or outlet manifolds respectively. The pressure in the inlet manifold is equal to or slightly less than the evaporator pressure. Similarly the pressure in the outlet manifold is equal to or slightly greater than the condenser pressure. The purpose of the manifolds is to provide stable inlet and outlet pressures for the smooth operation of the valves and also provide a space for mounting the valves. Rotary compressors The rotary compressors have replaced many smaller reciprocating compressors. A rotary unit is much smaller and lighter weight than a comparable reciprocating unit. A typical two blade rotary compressor operates as follows: As the first blade passes the suction port, low pressure vapour is drawn into the compressor When the second blade passes the suction port, it seals the refrigerant between the two blades, then starts a new cycle behind as it moves along. The gas is trapped and compressed as the blades revolves. As the first blade reaches the exhaust port, it pushes the compressed gas into the exhaust port. This entire cycle repeats itself as the blade rotate between intake and exhaust ports of the compressor. Rotary compressor Rotary compressors can have up to eight blades. This type of compressor is often used as the first or booster compressor in a cascade refrigeration system. They also provides large opening into the suction line, so that substantial amounts of vapour can be drawn in on each stroke maximizing the efficiency of the compressor. Small clearance used in the construction of rotary compressors ensures that low pressure vapour drawn into the unit is compressed and pushed out on the discharge stroke. A check valve is normally installed in the suction line to prevent high pressure vapour and compressor oil from flowing back into the evaporator. Rotary compressors Rotary vane compressor (courtesy: Hicks, Hargreaves & Co. Ltd) Screw compressors In the screw compressor, the screw comprises a set of precision –matched helical rotor to trap and compress refrigerant vapour as they turn inside a precisely machined compressed cylinder. The drive or the male rotor is driven by the compression motor. The lobes on the rotor fit into the interlobe spaces on the female rotor. As the rotor turns, low pressure vapour is drawn into the intake side of the compressor, filling the interlobe spaces. As the screw turn, the vapour is trapped, compressed and then discharged from the outlet or exhaust port. Operation is continuous and extremely smooth and vibration free. Oil injection is used on many screw compressors to help seal clearance between the rotors and the compression cylinder. Screw compressors are highly efficient. Screw compressors Describe the design and operational principles of single and twin screw compressors? Twin screw compressor (courtesy: Stal Refrigeration AB) Roto-dynamic compressors In roto-dynamic compressors, the pressure rise of refrigerant is achieved by imparting kinetic energy to a steadily flowing stream of refrigerant by a rotating mechanical element and then converting into pressure as the refrigerant flows through a diverging passage. Unlike positive displacement type, the rotodynamic type compressors are steady flow devices, hence are subjected to less wear and vibration. Depending upon the construction, roto-dynamic type compressors can be classified into: i. Radial flow type, or ii. Axial flow type Roto-dynamic compressor Centrifugal compressors (also known as turbocompressors) are radial flow type, roto-dynamic compressors. These compressors are widely used in large capacity refrigeration and air conditioning systems. Axial flow compressors are normally used in gas liquefaction applications. Centrifugal compressors A centrifugal compressor resembles a long horizontal cylinder. A drive shaft is mounted inside the cylinder and a series of impellers are mounted to the shaft. The impeller spin very fast. The refrigerant vapour is drawn in through vanes near the centre of the impeller. As the impellers turns, vapour is thrown outward against the outer wall of the impeller housing. The force exerted on the vapour increases it pressure by a small, but significant. The pressurized vapour the impeller body through slots in the perimeter of the body exits. It is then picked up by the second impeller and the process is repeated, further increasing vapour pressure. Centrifugal compressors are used for some large commercial applications. Based on arrangement of compressor motor or external drive • Open type • Hermetic (or sealed) type • Semi-hermetic (or semi-sealed) type Open type compressors In open type compressors the rotating shaft of the compressor extends through a seal in the crankcase for an external drive. The external drive may be an electrical motor or an engine (e.g. diesel engine). The compressor may be belt driven or gear driven. Open type compressors are normally used in medium to large capacity refrigeration system for all refrigerants and for ammonia (due to its incompatibility with hermetic motor materials). Open type compressors are characterized by high efficiency, flexibility, better compressor cooling and serviceability. However, since the shaft has to extend through the seal, refrigerant leakage from the system cannot be eliminated completely. Hence refrigeration systems using open type compressors require a refrigerant reservoir to take care of the refrigerant leakage for some time, and then regular maintenance for charging the system with refrigerant, changing of seals, gaskets etc. Hermetic (Sealed) compressors In hermetic compressors, the motor and the compressor are enclosed in the same housing to prevent refrigerant leakage. The housing has welded connections for refrigerant inlet and outlet and for power input socket. As a result of this, there is virtually no possibility of refrigerant leakage from the compressor. All motors reject a part of the power supplied to it due to eddy currents and friction, that is, inefficiencies. Similarly the compressor also gets heatedup due to friction and also due to temperature rise of the vapor during compression. In Open type, both the compressor and the motor normally reject heat to the surrounding air for efficient operation. In hermetic compressors heat cannot be rejected to the surrounding air since both are enclosed in a shell. Hence, the cold suction gas is made to flow over the motor and the compressor before entering the compressor. This keeps the motor cool. The motor winding is in direct contact with the refrigerant hence only those refrigerants, which have high dielectric strength, can be used in hermetic compressors. The cooling rate depends upon the flow rate of the refrigerant, its temperature and the thermal properties of the refrigerant. If flow rate is not sufficient and/or if the temperature is not low enough the insulation on the winding of the motor can burn out and short-circuiting may occur. Hence, hermetically sealed compressors give satisfactory and safe performance over a very narrow range of design temperature and should not be used for off-design conditions. Hermetic (Sealed) compressors The COP of the hermetic compressor based systems is lower than that of the open compressor based systems since a part of the refrigeration effect is lost in cooling the motor and the compressor. However, hermetic compressors are almost universally used in small systems such as domestic refrigerators, water coolers, air conditioners etc, where efficiency is not as important as customer convenience (due to absence of continuous maintenance). In addition to this, the use of hermetic compressors is ideal in systems, which use capillary tubes as expansion devices and are critically charged systems. Hermetic compressors are normally not serviceable. They are not very flexible as it is difficult to vary their speed to control the cooling capacity. In some (usually larger) hermetic units, the cylinder head is usually removable so that the valves and the piston can be serviced. This type of unit is called a semi-hermetic (or semi-sealed) compressor. Hermetic compressors Semi-hermetic compressor Hermetic compressor Courtesy: Durham Bush Ltd and L’UniteHermetique S.A