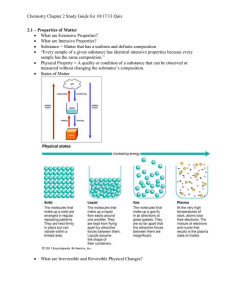
Matter—Properties and Change Section 3.1 Properties of Matter Section 3.2 Changes in Matter Section 3.3 Mixtures of Matter Section 3.4 Elements and Compounds Click a hyperlink or folder tab to view the corresponding slides. Exit Section 3.1 Properties of Matter • Identify the characteristics of a substance. • Distinguish between physical and chemical properties. • Differentiate among the physical states of matter. density: a ratio that compares the mass of an object to its volume Section 3.1 Properties of Matter (cont.) states of matter physical property solid extensive property liquid intensive property gas chemical property vapor Most common substances exist as solids, liquids, and gases, which have diverse physical and chemical properties. Substances • Matter is anything that has mass and takes up space. • Matter is everything around us. • Matter with a uniform and unchanging composition is a substance. States of Matter • The physical forms of matter, either solid, liquid, or gas, are called the states of matter. • Solids are a form of matter that have their own definite shape and volume. • Liquids are a form of matter that have a definite volume but take the shape of the container. States of Matter (cont.) • Gases have no definite shape or volume. They expand to fill their container. • Vapor refers to the gaseous state of a substance that is a solid or liquid at room temperature. Physical Properties of Matter • A physical property is a characteristic that can be observed or measured without changing the sample’s composition. Physical Properties of Matter (cont.) • Extensive properties are dependent on the amount of substance present, such as mass, length, or volume. • Intensive properties are independent of the amount of substance present, such as density. Chemical Properties of Matter • The ability of a substance to combine with or change into one or more other substances is called a chemical property. – Iron forming rust – Copper turning green in the air Observing Properties of Matter • A substance can change form–an important concept in chemistry. • Chemical properties can change with specific environmental conditions, such as temperature and pressure. Section 3.1 Assessment Density is what kind of property? A. atomic B. intensive C. extensive D C A 0% B D. dependent A. A B. B C. C 0% 0% 0% D. D Section 3.1 Assessment What defines a gas? A. Gases have a definite volume and shape. 0% A D. Gases have a definite shape but no definite volume. 0% B C. Gases have no definite volume or shape. A B C D 0% 0% D A. B. C. D. C B. Gases have a definite volume but take the shape of their container. Section 3.2 Changes in Matter • Define physical change and list several common physical changes. • Define chemical change and list several indications that a chemical change has taken place. • Apply the law of conservation of mass to chemical reactions. observation: orderly, direct information gathering about a phenomenon Section 3.2 Changes in Matter (cont.) physical change phase change chemical change law of conservation of mass Matter can undergo physical and chemical changes. Physical Changes • A change that alters a substance without changing its composition is known as a physical change. • Examples: crumpling, cutting, phase changes. • A phase change is a transition of matter from one state to another. • Boiling, freezing, melting, condensing and sublimation all describe phase changes in chemistry. Chemical Changes • A change that involves one or more substances turning into new substances is called a chemical change. • Examples: Bubbling, Change of smell, light being given off. • Decomposing, rusting, exploding, burning, or oxidizing are all terms that describe chemical changes. Conservation of Mass • The law of conservation of mass states that mass is neither created nor destroyed in a chemical reaction, it is conserved. • The mass of the reactants equals the mass of the products. massreactants = massproducts Section 3.2 Assessment When one substances turns into another, what kind of change has taken place? A. chemical reaction B. physical reaction D A 0% C D. nuclear reaction A. A B. B C. C 0% 0% 0% D. D B C. extensive reaction Section 3.2 Assessment The law of conservation of mass states that: A. Matter can be created and destroyed. B. Matter can be created but not destroyed. 0% 0% D 0% A B C D C 0% A D. The products of a reaction must have the same mass as the reactants. A. B. C. D. B C. The products of a reaction always have a greater mass than the reactants. Section 3.3 Mixtures of Matter • Contrast mixtures and substances. • Classify mixtures as homogeneous or heterogeneous. • List and describe several techniques used to separate mixtures. substance: a form of matter that has a uniform and unchanging composition; also known as a pure substance Section 3.3 Mixtures of Matter (cont.) mixture distillation heterogeneous mixture crystallization homogeneous mixture sublimation solution chromatography filtration Most everyday matter occurs as mixtures—combinations of two or more substances. Mixtures • A mixture is a combination of two or more pure substances in which each pure substance retains its individual chemical properties. • A homogenous mixture is a mixture where the composition is constant throughout. Mixtures (cont.) • Homogeneous mixtures are also called solutions. • A heterogeneous mixture is a mixture where the individual substances remain distinct. Mixtures (cont.) Separating Mixtures • Filtration is a technique that uses a porous barrier to separate a solid from a liquid in a heterogeneous mixture. • Distillation is a separation technique for homogeneous mixtures that is based on the differences in boiling points of substances. • Crystallization is a separation technique for homogenous mixtures that results in the formation of pure solid particles from a solution containing the dissolved substance. Separating Mixtures (cont.) • Sublimation is the process of a solid changing directly to a gas, which can be used to separate mixtures of solids when one sublimates and the other does not. • Chromatography is a technique that separates the components of a mixture on the basis of tendency of each to travel across the surface of another material. Section 3.3 Assessment Which is NOT a technique for separating a homogenous mixture? A. crystallization B. distillation D A 0% C D. chromatography A. A B. B C. C 0% 0% 0% D. D B C. filtration Section 3.3 Assessment Which of the following is a heterogeneous mixture? A. seawater B. silver mercury amalgam D A 0% C D. salad dressing A. A B. B C. C 0% 0% 0% D. D B C. atmosphere Section 3.4 Elements and Compounds • Distinguish between elements and compounds. • Describe the organization of elements in the periodic table. • Explain how all compounds obey the laws of definite and multiple proportions. proportion: the relation of one part to another or to the whole with respect to quantity Section 3.4 Elements and Compounds (cont.) element law of definite proportions periodic table percent by mass compound law of multiple proportions A compound is a combination of two or more elements. Elements • An element is a pure substance that cannot be separated into simpler substances by physical or chemical means. • 92 elements occur naturally on Earth. • Each element has a unique name and a one, two, or three-letter symbol. • The periodic table organizes the elements into a grid of horizontal rows called periods and vertical columns called groups. Compounds • A compound is a made up of two or more elements combined chemically. • Most of the matter in the universe exists as compounds. • Table salt, NaCl, and water, H2O, are compounds. Compounds (cont.) • Elements can never be separated. • Compounds can be broken into components by chemical means. Compounds (cont.) • This figure shows electrolysis of water to form hydrogen and oxygen. Compounds (cont.) • The properties of a compound are different from its component elements. Law of Definite Proportions • The law of definite proportions states that a compound is always composed of the same elements in the same proportion by mass, no matter how large or small the sample. Law of Definite Proportions (cont.) • The relative amounts are expressed as percent by mass, the ratio of the mass of each element to the total mass of the compound expressed as a percentage. Law of Definite Proportions (cont.) • This table demonstrates that the percentages of elements in sucrose remain the same despite differences in sample amount. Law of Multiple Proportions • The law of multiple proportions states that when different compounds are formed by a combination of the same elements, different masses of one element combine with the same relative mass of the other element in whole number ratios. – H2O2 and H2O – Copper(I) chloride and copper(II) chloride Law of Multiple Proportions (cont.) Section 3.4 Assessment What is a period on the periodic table of the elements? A. a vertical columns B. even numbered elements only D A 0% C D. the last vertical column only A. A B. B C. C 0% 0% 0% D. D B C. horizontal rows Section 3.4 Assessment An element is a substance that cannot be A. divided into simpler substances. B. combined to form a mixture. C. combined to form an element. D C A 0% B D. different phases. A. A B. B C. C 0% 0% 0% D. D Chemistry Online Study Guide Chapter Assessment Standardized Test Practice Image Bank Concepts in Motion Section 3.1 Properties of Matter Key Concepts • The three common states of matter are solid, liquid, and gas. • Physical properties can be observed without altering a substance’s composition. • Chemical properties describe a substance’s ability to combine with or change into one or more new substances. • External conditions can affect both physical and chemical properties. Section 3.2 Changes in Matter Key Concepts • A physical change alters the physical properties of a substance without changing its composition. • A chemical change, also known as a chemical reaction, involves a change in a substance’s composition. • In a chemical reaction, reactants form products. • The law of conservation of mass states that mass is neither created nor destroyed during a chemical reaction; it is conserved. massreactants = massproducts Section 3.3 Mixtures of Matter Key Concepts • A mixture is a physical blend of two or more pure substances in any proportion. • Solutions are homogeneous mixtures. • Mixtures can be separated by physical means. Common separation techniques include filtration, distillation, crystallization, sublimation, and chromatography. Section 3.4 Elements and Compounds Key Concepts • Elements cannot be broken down into simpler substances. • Elements are organized in the periodic table of the elements. • Compounds are chemical combinations of two or more elements and their properties differ from the properties of their component elements. Section 3.4 Elements and Compounds Key Concepts (cont.) • The law of definite proportions states that a compound is always composed of the same elements in the same proportions. • The law of multiple proportions states that if elements form more than one compound, those compounds will have compositions that are whole-number multiples of each other. Which of the following is NOT a physical property of water? A. Ice melts at 0°C. B. Water boils at 100. D A 0% C D. Water is a liquid at room temperature. A. A B. B C. C 0% 0% 0% D. D B C. Water reacts violently with pure sodium. 28.0 grams of nitrogen gas reacts completely with 6.0 grams of hydrogen to form 34.0 grams of ammonia. What does this demonstrate? A. the law of conservation of energy A 0% 0% A B C D 0% 0% D D. the law of conservation of mass C C. distillation A. B. C. D. B B. sublimation What is the best way to separate salt dissolved in water? A. sublimation B. crystallization D A 0% C D. filtration A. A B. B C. C 0% 0% 0% D. D B C. freezing Two or more elements chemically joined form what? A. substance B. heterogeneous mixture D A 0% C D. compound A. A B. B C. C 0% 0% 0% D. D B C. homogenous solution What is the ratio of oxygen to carbon in carbon dioxide (CO2)? A. 2:1 B. 1:2 D A 0% C D. 1:3 A. A B. B C. C 0% 0% 0% D. D B C. 1:1 Which is NOT a chemical reaction? A. a car rusting B. dissolving sugar in water C. wood burning D C A 0% B D. a banana ripening A. A B. B C. C 0% 0% 0% D. D Which describes a substance that is in the liquid state? A. It has a definite shape. B. It has no definite volume. D A 0% C D. It has a definite volume. A. A B. B C. C 0% 0% 0% D. D B C. It can be compressed into a smaller volume. Elements in the same period are likely to have similar ____. A. physical properties B. densities D A 0% C D. melting points A. A B. B C. C 0% 0% 0% D. D B C. chemical properties Filtration is an easy way to separate what? A. heterogeneous mixture B. homogeneous mixture D A 0% C D. solutions A. A B. B C. C 0% 0% 0% D. D B C. compounds Compounds can be broken into their component elements by which of the following? A. crystallization A 0% D D. chemical reaction C C. filtration A. A B. B C. C 0% 0% 0% D. D B B. distillation Click on an image to enlarge. Figure 3.4 Three Common States of Matter Figure 3.10 Conservation of Mass Table 3.3 Types of Solution Systems Click any of the background top tabs to display the respective folder. Within the Chapter Outline, clicking a section tab on the right side of the screen will bring you to the first slide in each respective section. Simple navigation buttons will allow you to progress to the next slide or the previous slide. The Chapter Resources Menu will allow you to access chapter specific resources from the Chapter Menu or any Chapter Outline slide. From within any feature, click the Resources tab to return to this slide. The “Return” button will allow you to return to the slide that you were viewing when you clicked either the Resources or Help tab. To exit the presentation, click the Exit button on the Chapter Menu slide or hit Escape [Esc] on your keyboards while viewing any Chapter Outline slide. This slide is intentionally blank.