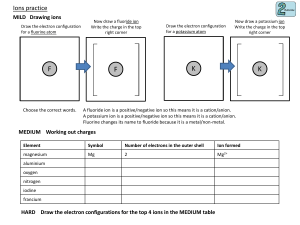
Learning Targets Unit 3 & 4 I can identify the location, charge and relative mass of each subatomic particle (electron, neutron and proton). I can identify the element based on the number of protons. I can describe how the model of the atom has changed over time. I can explain how ions are formed and predict their charge. I can explain how the number of protons and neutrons relates to the mass number. I can define isotope by explaining that atoms of an element can obtain different numbers of neutrons. I can count the number of atoms, molecules, or formula units in a substance. I can define the mole in terms of 6.02 x 1023 (Avogadro’s number) particles. I can describe the location of an electron in an orbital diagram. I can write the electron configuration of an atom or ion. I can predict the number of valence electrons in an atom or ion. I can explain the octet rule as it relates to ion formation.