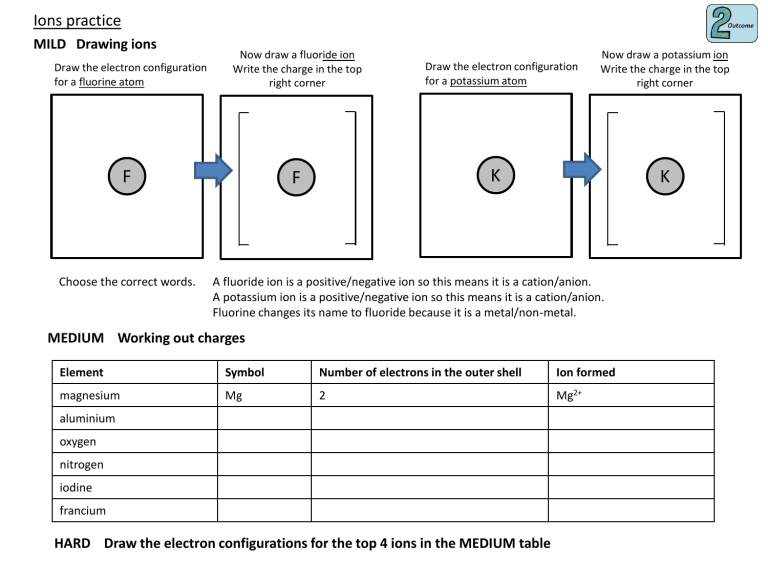
Ions practice MILD Drawing ions Draw the electron configuration for a fluorine atom Now draw a fluoride ion Write the charge in the top right corner F Choose the correct words. Draw the electron configuration for a potassium atom Now draw a potassium ion Write the charge in the top right corner K F K A fluoride ion is a positive/negative ion so this means it is a cation/anion. A potassium ion is a positive/negative ion so this means it is a cation/anion. Fluorine changes its name to fluoride because it is a metal/non-metal. MEDIUM Working out charges Element Symbol Number of electrons in the outer shell Ion formed magnesium Mg 2 Mg2+ aluminium oxygen nitrogen iodine francium HARD Draw the electron configurations for the top 4 ions in the MEDIUM table



