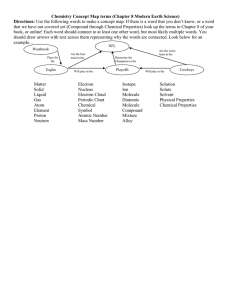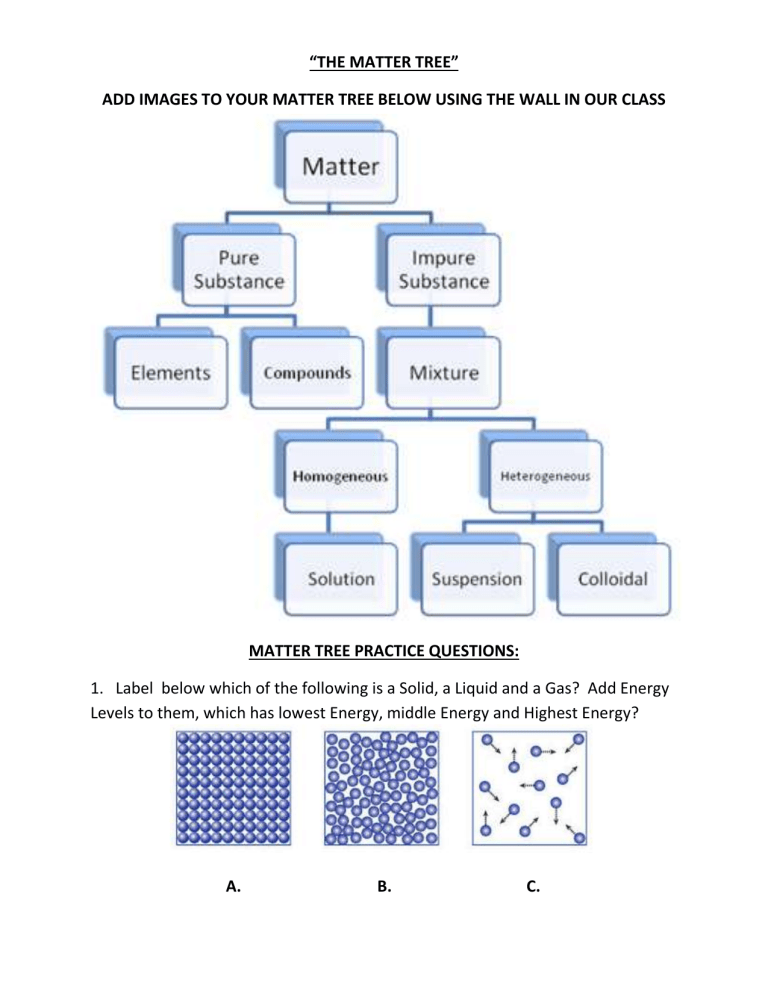
“THE MATTER TREE” ADD IMAGES TO YOUR MATTER TREE BELOW USING THE WALL IN OUR CLASS MATTER TREE PRACTICE QUESTIONS: 1. Label below which of the following is a Solid, a Liquid and a Gas? Add Energy Levels to them, which has lowest Energy, middle Energy and Highest Energy? A. B. C. 2. Define Density:___________________________________________________ A B C 3. Put in order of Low Density to High Density A, B, and C from the above picture. 4. Use “The Matter Tree” and words to describe the following picture. Identify what type of Matter it is? 5. Use “The Matter Tree” and words to describe the following picture. Identify what type of Matter it is? 6. Use “The Matter Tree” and words to describe the following picture. Identify what type of Matter it is? 7. Use “The Matter Tree” and words to describe the following picture. Identify what type of Matter it is? 8. Use “The Matter Tree” and words to describe the following picture. Identify what type of Matter it is? 9. 10. Use “The Matter Tree” and words to describe the following picture. Identify what type of Matter it is? 11. Draw me a Heterogeneous Mixture of a Diatomic Element and a 2 atom Compound 12. Use “The Matter Tree” and words to describe the following picture. Identify what type of Matter it is? Use words to describe A and B below: A B 13. Draw a Suspension 14. What is the difference between a Solution and a Colloid with Light? 15. Draw a Heterogeneous Mix of 2 Gas Diatomic Elements 16. What is this? 17. Which One is Less Dense? 18. What color is the Solute and what color is the Solvent in Both of the pictures? 19. Based on the prior picture, what do you think the difference is between a Solute and a Solvent? 20. What is the most widely used Solvent? Also known as the Universal Solvent. 21. The element Iodine has a greater density in its solid state then in its gaseous state. Also, Iodine exists as a diatomic element: I2 Draw a model of gaseous Iodine, I2 and solid Iodine, I2. 22. A molecule is the smallest chunk of matter you can have when dealing with bonded things such as Compounds or Diatomic Elements. An atom is the smallest chunk of matter you can have when dialing with Elements. Knowing this, which of the following below are atoms and which are molecules? 23. Below is a picture of an Ionic Salt Crystal chunk, NaCl or Sodium Chloride, also known as table salt. Based on the prior question, draw only one molecule of Sodium Chloride. 24. Below is a picture of Rock Candy, C6H12O6 or Glucose, also known as table sugar. Based on the prior question, Circle only one molecule of Glucose in the bottom picture which shows lots of Glucoses together. 25. Draw one molecule of diatomic Oxygen that you breathe. 26. Draw one molecule of water, H2O, that you drink. 27. Draw 3 molecules of diatomic Nitrogen that you also breathe. 28. Draw 2 molecules of Carbon Dioxide, CO2, that you exhale when breath 29. Draw a 4 atom Compound that contains 3 atoms of one type of element (use black) and 1 atom of a different element (use red). 30. Draw 3 molecules of the prior Compound. 31. In Salt Water (Sodium Chloride in Water), what is the Solute and what is the Solvent? 32. What is this? 33. Use “The Matter Tree” and words to describe the following picture. Identify what type of Matter it is? 34. Use “The Matter Tree” and words to describe the following picture. Identify what type of Matter it is? 35. Anything that occupies space and has a mass is called __________________. 36. What are these?