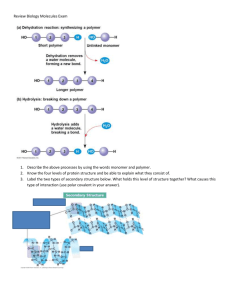
Carbohydrates Carbohydrates include individual sugar molecules and complex chains of sugar molecules. They are all composed of carbon, oxygen and hydrogen. Carbohydrates are the source of energy for all living things. Some carbohydrates also provide structural support. Monosaccharides (“monomers” _____________________________________) Chemical formula: _______________ Chemical formula: _______________ Chemical formula: _______________ Glucose, galactose and fructose are isomers of each other. What does that mean? Draw the skeletal structure for these molecules below. (Please note that in any evaluation for this unit, even a skeletal structure of a molecule must include show all hydrogen atoms.) There exist two isomers of glucose: α-glucose (alpha) and β-glucose (beta). Identify the difference between their structures. Humans can easily digest α-glucose starches, but not β-glucose polysaccharides (found in cellulose of plants). Disaccharides (“dimers” ___________________________________________) For two monosaccharides to form a disaccharide, a _________________________ reaction occurs. The bond formed between any two monosaccharides is called a glycosidic bond. For each of the following examples, draw the reaction with molecular structures below. Ex. α-glucose + α-glucose maltose + ________ Ex. α-glucose + fructose sucrose + ________ Ex. α-glucose + galactose lactose + ________ The glycosidic bond in maltose and sucrose is an α-linkage. The configuration of the glycosidic bond in lactose is different. This is a β-linkage. Polysaccharides (“polymers” ________________________________________) For each of the following examples, draw the reaction with molecular structures below. Ex. chain of β-glucose monomers is cellulose Ex. chain of α-glucose molecules is amylose (in starch) Ex. multiple chains of α-glucose form glycogen; to join chains, dehydration occurs between C1 hydroxyl group on last monomer of chain A and C6 hydroxyl group on any monomer of chain B. Wrap-Up Questions 1. Which type of carbohydrate molecule (mono-, di-, or polysaccharide) would you expect to taste sweetest? Why? 2. Refer to the terms “monomer” and “polymer” above. What do you suppose “polymerization” means? How does it apply to carbohydrates? 3. a) Draw the reaction that would break sucrose into its monosaccharide subunits. b) Draw the reaction that would break lactose into its monosaccharide subunits. c) What is this type of reaction called? 4. Carbohydrates are all polar molecules (because they are asymmetrical). a) Do you suppose monosaccharides are soluble in water? Why or why not? b) Do you suppose large polysaccharides (like those shown below) are soluble in water? Why or why not?