Building Carbohydrates Activity
advertisement

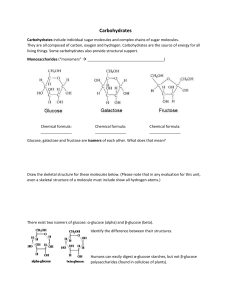
Carbohydrate activity for AP Biology Obtain an organic molecule kit. There should be 6 black (carbon), 6 red (oxygen), one or two blue (nitrogen or phosphorous), and a bunch of white (hydrogen). There should also be numerous gray sticks to connect them. Please record exactly the number that you have of each of the balls. Create a glucose ring structure without help Without looking into iPad yet, make a glucose molecule out of six carbons and one oxygen. Once the ring structure is complete, each remaining carbon should have an –H and –OH on the two remaining holes. The number 5 carbon should only have a hydrogen as its oxygen is the one in the ring itself. Check for position of –OH and –H relative to structure using iPad Now open iPad to any diagram of glucose that you want. Make sure it is the α-glucose and not the β-glucose version (yet). Notice how the –OH’s and the –H’s are not all on the same side of the structure. When viewing a two dimensional diagram, the –OH and – H facing up are up and those facing down are down when laying the molecule flat on the table. (The bottom of the two dimensional structure on the screen is facing you when flat on the table.) Now see how an alpha-glucose is different from a beta-glucose. What is the significant difference(s)? The main difference between α-glucose and β-glucose are in the types of molecules they make. When linking an α-glucose to another α-glucose then the molecules all face up (carbon 6 is up) which makes starch and glycogen. When linking an β-glucose to another α β-glucose then the molecules alternate face up and face down (carbon 6 is up then down) which makes cellulose. Making starch and/or glycogen Make all of your molecules α-glucose and combine them through condensation synthesis to make one long chain. Carbon 1 must ALWAYS be to the right when combining two glucose molecules and they will link to the 4 carbon of the next molecule. These bonds are always a 1-4 glycosidic linkages when joining two glucose molecules.Notice how all of the carbon 6’s of the glucose are facing upward on the table? Take your glucose out of the starch and replace the –OH or –H that you removed from it. Making cellulose Now convert all of your α-glucose into β-glucose. Do the same thing with other groups and combine the molecules. Carbon 1 must ALWAYS be to the right when combining two glucose molecules. These bonds are always a 1-4 glycosidic linkages when joining two glucose molecules. Notice how the only way to join them is to flip every other one of the molecules over to keep the strand straight. This is why it is impossible for animals to digest cellulose fibers because we have no enzymes to digest β-glycosidic bonds. Take your glucose out of the starch and replace the –OH or –H that you removed from it. Making Fructose Take your glucose molecule and break the bond between the oxygen in the ring and carbon 1. Remove the hydrogen from carbon 2 and place it on the open hole of carbon 1. Connect the oxygen to carbon 2. Open your iPad and find the structure of fructose. Does your structure look EXACTLY like the fructose in the diagram? If not, what would you have to change to make it match exactly. Make that change if you have to. Making Sucrose Pair up with another group. One group return the molecule to its original glucose structure. You will now do condensation synthesis to join the two molecules except this time you will form a 1-2 glycosidic linkage. You will have to turn the fructose around and face the table when you join them together. Notice how the carbon 1 is sticking up between the two sugar monomers when you form the dimer. Take your glucose/fructose out of the sucrose molecule and replace the –OH or –H that you removed from it. Take apart your molecule and put it back into the kit that it came from. Please check the number of each atom in the kit to assure me that there are none missing in your or another groups kit.