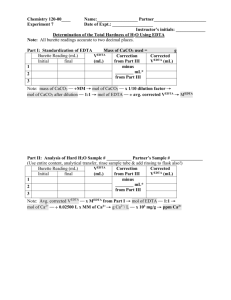
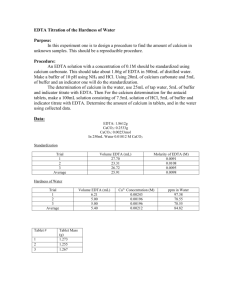
EDTA Titration Calculations The hardness of water is due in part to the presence of Ca2+ ions in water. The concentration of Ca2+ ions is usually expressed as ppm CaCO3 in the water sample. This is equivalent to 1 gram of CaCO3 in 10 6 grams of sample. In the lab 1 ppm CaCO3 is expressed as 1 mg CaCO3 per 1 Liter of sample or ppm is mg CaCO3 per L of sample. Calcium ions can be analyzed by titration with EDTA using an appropriate indicator. EDTA is ethylene diamine tetraacetic acid or H4C10H12N2O4. EDTA itself is not very water soluble so the disodium salt is used, Na2H2C10H12N2O4. For the purpose of simplicity, Y will stand for C10H12N2O4. The EDTA we use is thus Na 2H2 Y. The part that reacts with calcium ions is H2Y2- according to the following equation. H2Y2- + Ca2+ → CaY2- + 2H+ The procedure is done in 3 steps 1) Preparation of a standard Ca2+ solution, 2) Standardization of EDTA with the standard calcium solution and, 3) Analysis of an unknown Ca2+ sample with the standardized EDTA solution. 1. Standard calcium solution. 0.405 g of CaCO3 is dissolved in HCl and diluted to a volume of 250.00 mL. What is the molarity of calcium ions in the solution? CaCO3(s) + 2HCl(aq) → Ca2+ (aq) + 2Cl1-(aq) + H2O(l) + CO 2(g) 1molCaCO3 1molCa 2 100 . 09 gCaCO 1molCaCO3 3 0.405gCaCO3 2+ = 0.004505 mol Ca ions 0.00405molCa 2 Molarity of Ca = = 0.0162 M Ca2+ ions 0.250 L 2+ 2. Standardization of EDTA. A 25.00 mL aliquot (a portion or part) of the standard calcium solution reacts with 27.25 mL of the EDTA solution. What is the molarity of the EDTA? 0.0162molCa 2 1molEDTA 0.02500L = 0.000405 mol EDTA 2 L 1 1molCa Molarity of EDTA = 0.000405molEDTA = 0.0149 M EDTA 0.02725L 1 3. Ca2+ concentration in an unknown solution CaCO 3 reported as ppm CaCO3. A 50.00 mL unknown solution requires 25.55 ml of the standardized EDTA solution for complete reaction. What is the Ca2+ concentration in the unknown reported as ppm CaCO3 ? 1molCa 2 0.0149molEDTA 0.02555L = 0.000381mol Ca2+ L 1 1 molEDTA 1molCaCO3 0.000381molCa 2 2 1molCa ppm CaCO3 = 100.09 gCaCO3 1molCaCO = 0.0381g CaCO3 3 1000 mgCaCO3 1 = 0.0381gCaCO3 = 762 LSolution 0.05000LSolution 1.000 gCaCO3 mgCaCO 3 2