Catalase Enzyme Lab Activity: Effects of Temperature, pH, Salinity
advertisement

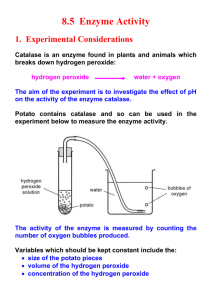
Catalase Lab Activity Introduction Enzymes are biological catalysts that help to carry out the thousands of chemical reactions that occur in living cells. They are generally large proteins made up of several hundred amino acids. In an enzyme-catalyzed reaction, the substance to be reacted, the substrate, binds to the active site of the enzyme. The enzyme then converts the substrate to products. Finally, the products are released into solution and the enzyme is ready to help with another reaction. As is true of any catalyst, the enzyme is not used up as it carries out the reaction but is recycled again and again. One enzyme molecule can carry out thousands of reaction cycles every minute!! Each enzyme is specific for a certain reaction because its amino acid sequence is unique and causes it to have a unique three-dimensional structure. The "business" end of the enzyme molecule, the active site, also has a specific shape. Because the active site is so specific it will only bind with one kind of molecule. An enzyme can be denatured (unfolded) by extreme heat, pH, or ionic concentration. If this happens, the enzyme will no longer be functional because the shape of the active site will be destroyed. In this exercise you will study the enzyme catalase, which speeds up the breakdown of hydrogen peroxide, H2O2, (a common waste product of cellular metabolism) into water and oxygen. The reaction is: It’s important for cells to be able to break down hydrogen peroxide as soon as it’s generated in the cell because hydrogen peroxide is quite toxic for cells. The products of the reaction, water and oxygen, are not toxic for the cell. So catalase helps to protect the cell from damage that could be caused by its own metabolic waste. Catalase is a very important enzyme! Because it’s so important, catalase is found in many animal and plant tissues. Pre Lab Questions: 1. What is the reactant (substrate) in the reaction above?___________ 2. What are the products in the reaction above? ___________________________ 3. Would this reaction happen by itself without the help of catalase? Explain. PART A - Observe Normal Catalase Reaction 1. Place about 2 ml of the 3% hydrogen peroxide solution into a clean test tube. 2. Using forceps and scissors cut a small piece of liver and add it to the test tube. Push it into the hydrogen peroxide with a stirring rod. Observe the bubbles. What gas is being released? (consider the equation above—on the first page) _____________________ Throughout this investigation you will estimate the rate of the reaction (how rapidly the solution bubbles) on a scale of 0-5 (0=no reaction, 1=slow, ..... 5= very fast). Assume that the reaction in step 2 proceeded at a rate of "4" Assume that the normal reaction rate of catalase is a 3. Recall that a reaction that absorbs heat is endothermic; a reaction that gives off heat is exothermic. Feel the temperature of the test tube with your hand. Has it gotten warmer or colder ____________ Is the reaction endothermic or exothermic __________________ 3. Pour off the liquid into a second test tube. Assuming the reaction is complete, what is this liquid composed of? (refer to the equation on page 1 again) _________________ What do you think would happen if you added more liver to this liquid? ______________________ Test this (add more liver) and record the reaction rate. Reaction Rate ___________ (1 – 5) 4. Add 2 more mL of hydrogen peroxide to the liver remaining in the first test tube. What is the reaction rate? (1 – 5)______ Is catalase reusable? Explain how you know. Part B - What Tissues Contain Catalase? You will now test for the presence of catalase in tissues other than liver. Place 2 ml of hydrogen peroxide in each of 3 clean test tubes and then add each of the three test substances to the tubes. As you add each test substance, record the reaction rate (0-5) for each tube in the data table. Substance Rate of Reaction (0-5) Does tissue contain Catalase?-YES OR NO If YES in column 3, how can you tell it contains catalase? Potato Apple Carrot PART C - What is the Effect of Temperature on Catalase Activity? 1. Put a piece of liver into the bottom of a clean test tube and cover it with a small amount of water. Place this test tube in a boiling water bath for 5 minutes. 2. Remove the test tube from the hot water bath, allow it to air cool, then pour out the water. Add 2 ml of hydrogen peroxide. CAUTION: Use a test-tube holder for hot test tubes. What is the reaction rate for the boiled liver and peroxide? (O-5). ____________ 3. Put a small quantity of liver into 1 other clean test tube and 1 ml H2O2. into another test tube. Put this test tube of liver and one of H2O2 into an ice bath. After 3 minutes, pour the tube of H2O2 into the corresponding tube of liver and observe the reaction What is the reaction rate for the cold liver/peroxide? (O-5). _____ PART D - What is the Effect of pH on Catalase Activity 1. Add 2 ml hydrogen peroxide to 3 clean test tubes, then add: Tube 1—add 1 ml acetic acid (vinegar) pH=2.6 Tube 2 – add 1 ml sodium bicarbonate solution (baking soda solution) (base) pH = 9 Tube 3 – add 1 ml distilled water (neutral) pH = 7 Now add liver to each of the test tubes (try to do it all at about the same time, so you can easily compare) Rate of Reaction for: (0-5) Acid _____ Neutral _____ Base_____ Part E-What is the Effect of Salinity (high salt conc.) on Catalase Activity. 1. Label a test tube “SP” for “Salty potato puree.” Use a spatula to take A SMALL AMOUNT (about 5 chunks) of your potato puree and transfer it into the “SP” test tube. Push it down to the bottom of the test tube with a stirring rod. 2. Use a pipet to add 10 drops of salt solution to the SP test tube. 4. Use a pipet and measure 3ml of hydrogen peroxide. Place the hydrogen peroxide into the “SP” test tube. 5. Record your observations on the data sheet provided. Rate of Reaction for: (0-5) _________ DATA ANALYSIS 1). Describe the relationship between catalase and hydrogen peroxide. Indicate which is the enzyme, which is the substrate and what occurs during the reaction. 2). Is catalase reusable? Use your data to support your answer. 3A). How does temperature and pH affect the reaction rate of catalase? 3B). Explain what happened to the catalase at the molecular level. 4). In 2-3 sentences, summarize the data and information displayed in this graph. ! 5A). What were some independent variables in this lab? 5B). What was the dependent variable in this lab? 5C). List 3 constants: 5D). Positive Control= Negative Control= 6). Why was it necessary to puree the plant tissue in order to test for catalase activity? 7). Draw and label the following: enzyme catalase, the substrate hydrogen peroxide and the active site. Also draw and label the products formed from this reaction.