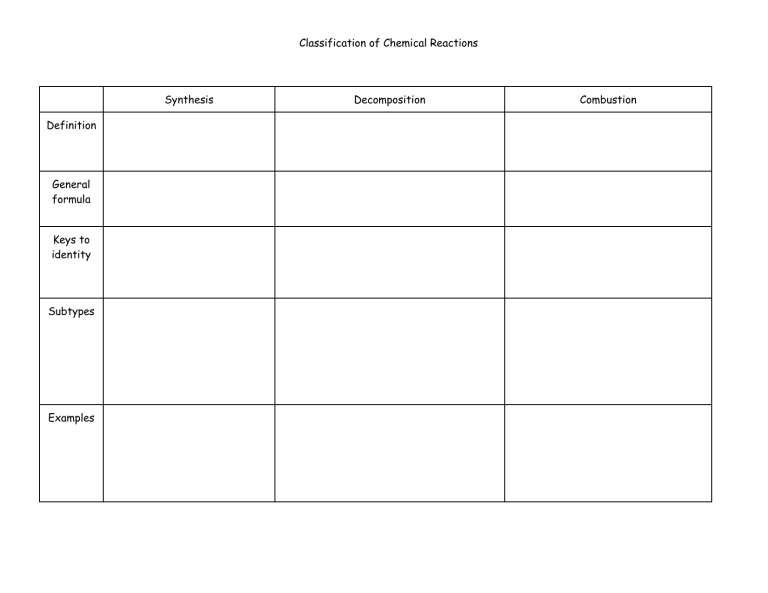
Classification of Chemical Reactions Synthesis Definition General formula Keys to identity Subtypes Examples Decomposition Combustion Other info Redox? Classification of Chemical Reactions Single replacement Definition General formula Keys to identity Subtypes Double replacement Examples Other info Redox? Rules for Assigning Oxidation Numbers RULE EXAMPLE 1.The oxidation number of any uncombined element is 0. Na, F2, S8 all = 0 2.The oxidation number of a monatomic ion equals the charge on the ion. CaCl2, Ca = +2 and Cl = -1 3.The more-electronegative element in a binary compound is assigned CCl4, Cl = -1, C = +4 the number equal to the charge it would have if it were an ion. 4.The oxidation number of fluorine in a compound is always -1. HF, F = -1, H = +1 5.Oxygen has an oxidation number of -2 unless it is combined CaO, O = -2, H2O, O = -2 with F (when it is +2), or it is in a peroxide when it is -1. 6.The oxidation state of hydrogen in most of its compounds is +1 unless it is combined with a metal, in which case it is -1. OF2, O= +2, H2O2, O= -1 HCl, H = +1, NaH, H = -1 7. The sum of the oxidation numbers of all atoms in a neutral compound is 0 NO, O = -2, so N = +2 because -2 + (+2) = 0 NO2, O= -2, so N = +4 because -2(2) + (+4) = 0 8.The sum of the oxidation numbers of all atoms in a polyatomic ion equals the charge of the ion. SO4-2, O = -2 so S = +6 because -2(4) = (+6) = -2 SO3-2, O = -2 so S = +4 because -2(3) = (+4) = -2 For each of the reactions below, assign oxidation numbers to all and identify what has been oxidized and what has been reduced. 4 C5H9O(g) + 27 O2(g) → 20 CO2(g) + 18 H2O(g) 2 Fe(s) + O2 (g) → 2 FeO (s) 2 NH3(aq)+ H2SO4(aq) → (NH4)2SO4(aq)






