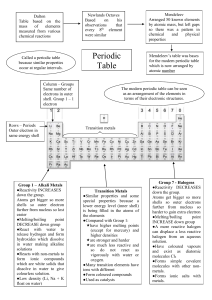
1 L1: The Periodic Table The periodic table is a remarkable way to show the manifold relationships between differing kinds of elements • The modern table was devised in 1869 by Dimitri Mendeleyev • He arranged the elements by weight and by their chemical properties The periodic table arranges all the elements in groups according to their properties. 2 Mendeleev The Periodic Table • To make the periodic table, the elements are first laid out in order of increasing Atomic/Proton Number • Based on the arrangements of the electrons outside the nucleus – Elements whose atoms have 1 outer-shell electron are picked out of the list in order and called Group 1 – Elements whose atoms have 2 outer-shell electron are picked out of the list in order and called Group 2. – Next: Group 3 followed by Group 4 and so on. • The Groups are arranged side by side to give the periodic table 3 4 Arrangement of Elements Vertically into columns called GROUPS Groups Horizontally Into rows called PERIODS Periods 6 Groups If you looked at one atom of every element in a group you would see… Each atom has the same number of electrons in it’s outermost shell. 7 Groups Example: The group 1 atoms all have 1 electron in their outer shells 8 Groups Example: The group 2 atoms all have 2 electrons in their outer shells Be (Beryllium) Atom Mg (Magnesium) Atom What is happening to the number of shells as you go down the 9 group? Groups Fact : Elements in the same group have the same number of electrons in the outer shell (this correspond to their group number) H e H Li B e B C N O F N e N a M g Al Si P S Cl Ar K C a Fe Ni C u Zn A g E.g. all group 1 metals have __ electron in their outer shell A Br Kr I H Pt These elements u have g __ electrons in their outer shells Xe These elements have __ electrons 10 in their outer shell The Periodic Table Fact : As you move down through the groups an extra electron shell is added: Li B e Na M g K C a E.g. Lithium has 3 H electron in the configuration 2,1 H e Ni Sodium hasFe11 electrons in the configuration 2,8,1 Potassium has 19 electrons in the configuration __,__,__ Pt C u Z n A g A u N e B C N O F Al Si P S Cl Ar Br Kr I X e H g 11 Periods If you looked at an atom from each element in a period you would see… Each atom has the same number of electron holding shells. What is happening to the number of electrons in the outermost shell? 12 Periods Eg: The period 4 atoms each have 4 electron containing shells 4th Shell K (Potassium) Kr (Krypton) Atom Atom 13 Fe (Iron) Atom 14 The groups • The periodic Table is divided into several groups based on the properties of different atoms. • The table has 8 groups of elements plus a block of transition metals. • Some of the groups have special names: – Group 1: alkali metals – Group 2: alkaline earth metals – Group 7: halogens – Group 0 or 8: noble gases 15 The groups • Groups can be separated into metals and non-metals • Transition metals: atoms have more complicated electron arrangements • Contains many common metals such as iron (Fe), Nickel (Ni) and copper (Cu). A group of elements is sometimes called a family, because its elements resemble each other. Sometimes they look alike and usually behave alike. 16 The Periodic Table Fact : Most of the elements are metals: H Li Be These elements are metals He Na Mg K Ca Fe Ni Cu Zn Ag Pt Au This line divides metals from nonmetals B C N O F Ne Al Si P S Cl Ar Br Kr I Xe Hg These elements are 17 non-metals Regions of the Periodic Table 18 Valence Electrons • The number of outer or “valence” electrons in an atom affects the way an atom bonds. • The way an atom bonds determines many properties of the element. • This is why elements within a group usually have similar properties. 19 Group 1: The Alkali Metals Alkali Metals •The first three elements of Group 1: lithium [2,1], sodium [2,8,1], potassium [2,8,8,1] – Soft metals that can be cut with a knife – So light that they float on water – Silvery & shiny when freshly cut; quickly tarnish – Have low melting & boiling points compared with other metals All react in a similar way sodium 20 Group 1: The Alkali Metals • They have similar properties because: – 1 electron in the outermost shell. – they have one more electron than a stable Noble Gas electron structure. – So, they readily lose the outer electron when they chemically react Trends within group 1: • Down the group: – Reactivity increases as atoms get larger–WHY? – Melting and boiling points decreasesWHY? 21 Group 2- the Alkaline earth metals Alkaline Earth Metals •Silvery metals •Fairly reactive –Less reactive compared with elements in group I •Many are found in rocks in the earth’s crust Magnesium Magnesium 22 oxide Group 2- the Alkaline earth metals Trends down the group Less reactive compared with elements in group 1; they have to give up two outer electrons to obtain a full outer shell. Reactivity increases down the group-WHY? M.ps and B.Ps generally decrease down the group. 23 Group 7: The Halogens Most common halogens: chlorine, bromine and iodine chlorine iodine Bromine 24 Group 7 –Halogens – Some uses salt makers 25 Group 7 –the Halogens • Halogens have similar properties because their atoms have 7 electrons in the outer shell. eg: fluorine [2,7], chlorine [2,8,7] • They are reactive because their atoms are just one electron short of a full outer shell. • They are diatomic because two atoms can gain full shells by sharing electrons with each other 26 Group 7 –the Halogens Trends within group 7: •Down the group: –Reactivity decreases as atoms get larger. –m.p and b.p increase because the attraction between the molecules increase. Gas Liquid solid 27 Group 7: Halogens & its uses Fluorine: • Fluorides are added to toothpaste and sometimes drinking water. It has been shown that fluorides can reduce dental decay (damage to teeth) especially in young children • It is used to make making refrigerants and detergents Chlorine: • The most common use of chlorine is in drinking water and swimming pool as it can kill harmful bacteria. • To ,manufacture insecticides, solvents, bleach, food paints, plastic, dyes, textiles, petroleum products, paper products etc. 28 Group 7: Halogens & its uses Bromine: • Bromine is also used to disinfect water as it can kill the bacteria present in the water. • The inorganic form of bromine is used in photography film, • It is also used in, dyes and medicines. Iodine: • The compounds are basically used in medicine, photography and dyes. • Is a good antiseptic to kill bacteria • Another very important use of iodine is as it is quite radio opaque, it can be used as X-ray radio contrast agent, for intravenous injection. • Is also an important dietary mineral 29 Group 8 – The noble (inert) gases • Non metals • Colourless mono-atomic gases • Very unreactive because they have full outer shells ( V stable) • All noble gases are not identical • Density of the gases increase down the group; because mass of atom increases 30 Colors Noble Gases produce in lamp tubes: • Ne (Neon): orange-red • Ar (Argon): pale lavender • He (Helium): pale peach • Kr (Krypton): pale silver • Xe (Xenon): pale, deep blue 31 Jellyfish lamps made with noble gases 32 Transition Metals • Most are good Conductors of electricity • Malleable (easily bent/hammered into wires or sheets) 33 Transition metals • • • • • • • Hard and dense High m.p Not very reactive Form coloured compounds Have variable valency Many are used in making alloys Many are used as catalysts Iron in air gives iron(III) oxide 34 Hydrogen • Stands on its own in the periodic table • It has one outer electron, like group 1 metals, but unlike them it is a gas • It usually reacts like a non metal 35 Trends across the periodic table • Number of outer shell electrons increase by one from one group to the next 37 Trends across the periodic table • The electron arrangements of the first 20 elements: • The number of outer shell electrons increase by 1 with each 38 group. General Periodic Trends • Atomic and ionic size • Metallic activity • Nonmetallic activity Higher effective nuclear charge Electrons held more tightly Larger orbitals. Electrons held less tightly. 39 Trends… 40 41 Atomic Size • Size goes UP on going down a group. • Because electrons are added further from the nucleus, there is less attraction. • Size goes DOWN on going across a period. 42 Atomic Radii 43 Ion Sizes + Li,152 pm 3e and 3p Li + , 78 pm 2e and 3 p Forming a cation. • CATIONS are SMALLER than the atoms from which they come. • The electron/proton attraction has gone UP and so size DECREASES. 44 Ion Sizes F, 71 pm 9e and 9p F- , 133 pm 10 e and 9 p Forming an anion. • ANIONS are LARGER than the atoms from which they come. • The electron/proton attraction has gone DOWN and so size INCREASES. • Trends in ion sizes are the same as atom sizes. 45