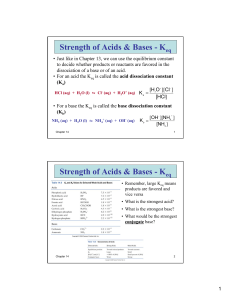
Chapter 6: Understanding Organic Reactions 1. Which of the following statements about substitution reactions is true? A) Substitution reactions involve p bonds. B) Substitution reactions involve s bonds. C) One s bond breaks and another forms at a different carbon atom. D) One p bond breaks and another forms at the same carbon atom. 2. What kind of reaction does the conversion of A to B represent? A) Addition reaction B) Substitution reaction. C) D) Elimination reaction. Acid-base reaction. Which of the following statements about elimination reactions is true? A) Two s bonds are broken. B) Two s bonds are formed. C) D) Two p bonds are broken. Two p bonds are formed. What kind of reaction does the conversion of A to B represent? A) Addition reaction. B) Elimination reaction. C) D) Substitution reaction. Oxidation-reduction reaction. Which of the following statements about addition reactions is true? A) Two p bonds are formed. B) Two p bonds are broken. C) D) Two s bonds are formed. One p bond is formed. What kind of reaction does the conversion of A to B represent? A) Acid-base reaction. B) Elimination reaction. C) D) Substitution reaction. Addition reaction. 3. 4. 5. 6. 7. Which of the following statements about bond breaking is true? A) Homolysis and heterolysis require energy. B) In homolysis, the electrons in the bond are divided unequally. C) In heterolysis, the electrons in the bond are divided equally. D) Homolysis generates charged intermediates. 8. Which of the following statements about bond breaking is not true? A) Homolysis generates uncharged reactive intermediates with unpaired electrons. B) Homolysis require energy but heterolysis does not require energy. C) Heterolysis generates charged intermediates. D) Heterolysis involves unequal sharing of bonding electrons by atoms. 9. Which of the following statements is true? A) Ionic intermediates are formed in radical reactions. B) Radicals are intermediates in polar reactions. C) Carbocations are electrophiles. D) Radicals are nucleophiles. 10. Which of the following statements is not true? A) In polar reactions, a nucleophile reacts with an electrophile. B) Carbocations are electrophiles. C) Carbanions are nucleophiles. D) A half-headed curved arrow shows the movement of an electron pair. 11. Which of the following statements is not true? A) Bond breaking is endothermic. B) The bond dissociation energy for bond breaking is always negative. C) Bond making is exothermic. D) The bond dissociation energy for bond formation is always negative. 12. Which of the following statements is true? A) Bond dissociation energies increase down a column of the periodic table. B) When DH° is positive, more energy is released in forming bonds than is needed to break bonds. C) When DH° is negative, more energy is needed to break bonds than is released in forming bonds. D) Bond dissociation energies decrease down a column of the periodic table. 13. Using the bond dissociation energies given, calculate DH° for the following reaction. Bond A-B (CH3)3C-Cl H-OH (CH3)3C-OH H-Cl A) +3 KJ/mol 14. DH° KJ/mol 331 498 401 431 B) -3 KJ/mol C) -67 KJ/mol D) +70 KJ/mol Using the bond dissociation energies given, calculate DH° for the following reaction. Bond A-B CH3CH2-Br H-OH CH3CH2-OH DH° KJ/mol 285 498 393 H-Br A) +108 KJ/mol 368 B) -130 KJ/mol C) -22 KJ/mol D) +22 KJ/mol 15. Which of the following statements about the equilibrium constant, Keq, is true? A) When Keq > 1, the equilibrium favors the reactants. B) When Keq < 1, the equilibrium favors the products. C) The size of Keq tells about the position of equilibrium. D) For a reaction to be useful, the equilibrium must favor the reactants. 16. Which of the following statements about equilibrium is true? A) Equilibrium favors the products when the energy of the products is higher than the energy of the reactants. B) Equilibrium favors the reactants when the energy of the product is lower than the energy of the reactants. C) Equilibrium favors the products when they are less stable than the starting material of a reaction D) Equilibrium favors the products when they are more stable than the starting material of a reaction. 17. Which of the following expressions summarizes the correct relationship between the free energy change, DG°, and the equilibrium constant, Keq? A) Keq > 1 when DG° > 0 C) Keq < 1 when DG° < 0 B) Keq > 1 when DG° < 0 D) Keq < 1 when DG° = 0 18. Which of the Keq corresponds to the lowest value of DG°? A) Keq = 10-3 B) Keq = 10-2 C) Keq = 10-1 D) DG° cannot be determined 19. Which of the Keq corresponds to the highest value of DG°? A) Keq = 10-1 B) Keq = 10-2 C) Keq = 10-3 D) Keq = 10-5 20. Which of the Keq corresponds to the most negative value of DG°? A) Keq = 1 B) Keq = 101 C) Keq = 102 D) Keq = 103 21. Which of the following statements is true? A) The product is favored in reaction in which DH° is a positive value. B) Entropy decreases when an acyclic compound forms a ring. C) In homolytic bond cleavage, entropy decreases and favors formation of products. D) The starting material is favored in a reaction in which DH° is a negative value. 22. Which of the following statements is true? A) The size of the activation energy tells us about the reaction mechanism. B) The size of the activation energy tells us about the reaction rate. C) A slow reaction has low activation energy. D) A fast reaction has large activation energy. 23. 24. 27. Which of the following statements is not true? A) Two reactions can have identical values for DH° but very different Ea values. B) The larger the activation energy, the slower the reaction. C) DH° determines the height of the energy barrier. D) The lower the activation energy, the faster the reaction. What is the name given to the reaction species that lies at an energy minimum between steps on a reaction energy diagram? A) Transition state C) Reactive intermediate B) Activation energy D) Equilibrium product 25. Which of the following statements about a two-step reaction mechanism is true? A) The transition states are located at energy minima. B) Each step is characterized by its own value of DH° and Ea. C) The rate-determining step has the lower energy transition state. D) The reactive intermediate is located at an energy maximum. 26. Which reaction is fast and has Keq= 1? A) A B) B C) C Which reaction has a positive DG°, assuming that entropy changes are negligible compared to enthalpy changes? A) A B) B C) C 28. Which reaction is slowest? A) A B) B C) C 29. In which reaction is Keq > 1? A) A B) B C) C 30. How many transition states are present in the reaction in the energy diagram? A) 0 B) 1 C) 2 D) 3 31. Which of the following letters represents DH° for the forward reaction in the following energy diagram? A) A B) B C) C D) D 32. How many transition states and intermediates would the reaction profile have for the reaction shown below? A) Three transition states and three intermediates B) Two transition states and two intermediates C) D) 39. 40. Three transition states and two intermediates Two transition states and three intermediates 33. Which step would most likely have the largest energy of activation? A) Step one B) Step two C) Step three D) It cannot be determined from the information provided 34. A decrease in which of the following results in an increase in the rate of a chemical reaction? A) Energy of activation B) Concentration C) Temperature D) Kinetic energy 35. Which of the following reaction quantities will have an effect on reaction rate? A) DG° B) DH° C) Keq D) Ea 36. Which of the following statements is true? A) Fast reactions have small rate constants. B) Slow reactions have large rate constants. C) A rate equation contains concentration terms for all reactants involved in a one-step mechanism. D) A rate equation contains concentration terms for all the reactants involved in a multi-step reaction. 37. The equilibrium constant for the conversion of A to D is predicted to be which of the following? A) Keq = 1 B) Keq < 1 C) Keq > 1 D) Cannot be determined from the information provided 38. The DG° (free energy change) for the conversion of A to B is predicted to be which of the following? A) DG° = 0 B) DG° < 0 C) DG° > 0 D) Cannot be determined from the information provided What kind of reaction does the conversion of A to D represent? A) Addition reaction B) Substitution reaction C) D) Elimination reaction Oxidation-reduction reaction If the conversion of A to B is slow and B to C is fast, what is the rate equation for this reaction? A) Rate = k[(CH3)2CHCl][H2O] C) Rate = k[(CH3)2CH]+[H2O] B) Rate = k[(CH3)2CHCl] D) Rate = k[(CH3)2CH]+ 41. Which compound would you predict to be highest in energy? A) A B) B C) C D) D 42. Calculate Ea for the conversion of C ® B. Ea (A ® B) = +10 kcal Ea (B ® C) = +4 kcal DH (A ® B) = +8 kcal DH (B ® C) = -5 kcal A) +3 kcal B) +7 kcal 43. C) +9 kcal D) None of the above The following is an energy diagram for the conversion of A ® B ® C. The energies of activation and DH’s for each step are also given. Calculate DH overall as shown on the energy diagram for A ® B ® C. Ea (A ® B) = +10 kcal Ea (B ® C) = +4 kcal DH (A ® B) = +8 kcal DH (B ® C) = -5 kcal A) +3 kcal B) +7 kcal C) +9 kcal D) None of the above 44. Which of the following statements about a catalyst is true? A) A catalyst accelerates a reaction by changing the amount of reactant and product at equilibrium. B) A catalyst accelerates a reaction by lowering the energy of activation. C) A catalyst accelerates a reaction by raising the energy of activation. D) A catalyst accelerates a reaction by lowering the equilibrium constant. 45. Which of the following statements about enzymes is true? A) Enzymes increase the activation energy for a reaction. B) Enzymes decrease the equilibrium constant. C) Enzymes shift the equilibrium to favor the product. D) Enzymes lower the transition state for the rate-determining step. Answer Key 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. 14. 15. 16. 17. 18. 19. 20. 21. 22. 23. 24. 25. 26. 27. 28. 29. 30. 31. 32. 33. 34. 35. 36. 37. 38. 39. 40. 41. 42. 43. B B A B C D A B C D B D B D C D B C D D B B C C B B A A C C D C A A D C C C B B B C A 44. 45. B D