Notes- Empirical & Molecular Formulas
advertisement
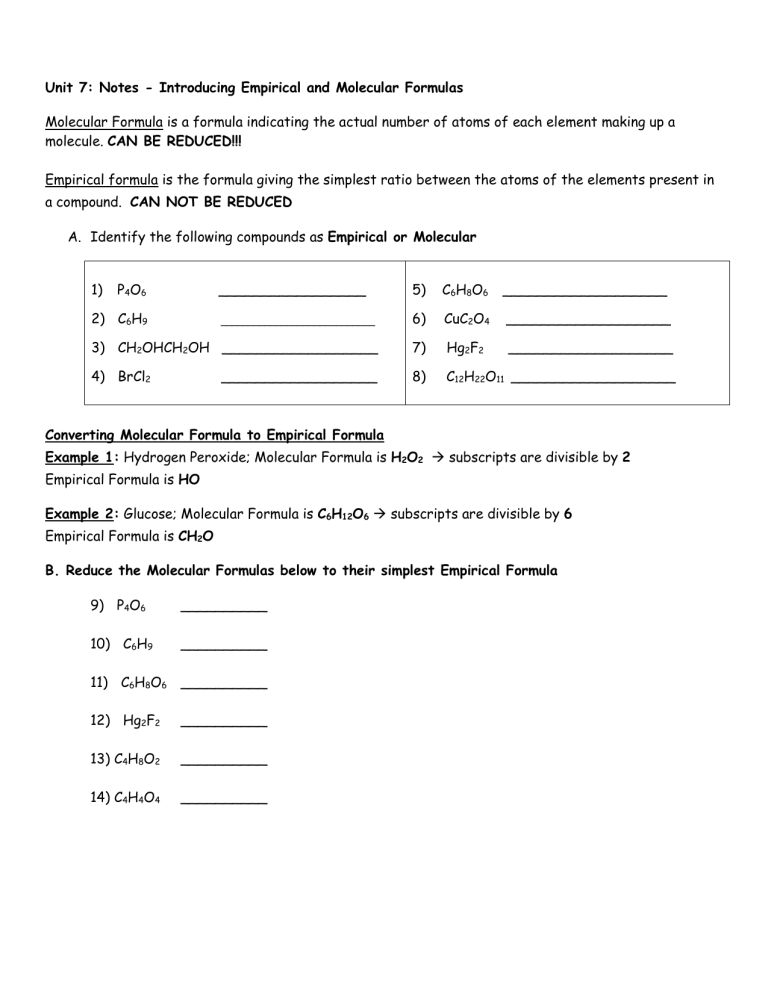
Unit 7: Notes - Introducing Empirical and Molecular Formulas Molecular Formula is a formula indicating the actual number of atoms of each element making up a molecule. CAN BE REDUCED!!! Empirical formula is the formula giving the simplest ratio between the atoms of the elements present in a compound. CAN NOT BE REDUCED A. Identify the following compounds as Empirical or Molecular 1) P4O6 _________________ 5) C6H8O6 ___________________ ____________________________ 6) CuC2O4 ___________________ 3) CH2OHCH2OH __________________ 7) Hg2F2 ___________________ 4) BrCl2 8) C12H22O11 ___________________ 2) C6H9 __________________ Converting Molecular Formula to Empirical Formula Example 1: Hydrogen Peroxide; Molecular Formula is H2O2 subscripts are divisible by 2 Empirical Formula is HO Example 2: Glucose; Molecular Formula is C6H12O6 subscripts are divisible by 6 Empirical Formula is CH2O B. Reduce the Molecular Formulas below to their simplest Empirical Formula 9) P4O6 __________ 10) C6H9 __________ 11) C6H8O6 __________ 12) Hg2F2 __________ 13) C4H8O2 __________ 14) C4H4O4 __________ Find the Empirical Formulas for the following: 15) A compound composed of 9.93% Carbon, 58.6% Chlorine and 31.4% Fluorine. Change % to g Gram to mole (divide by Molar mass) DO NOT ROUND!! Divide by smallest Multiply ‘till whole Or Round C Cl F Answer: the empirical formula is ______________________ 16) A compound is 30% Nitrogen and 70% Oxygen. Find the empirical formula of the compound. N O Answer: the empirical formula is ______________________ 17) Find the Molecular Formulas for the following compounds: 1. Find the molar mass of the empirical formula. 2. Divide the Given by the molar mass you found to get the value of n 3. Multiply the subscripts of the empirical formula by the value of n to get the molecular formula a) A compound with a molecular mass of 70 g and an empirical formula of CH2. b) A compound with a molecular mass of 46.0 g and an empirical formula of NO2.