Rate of reactions
advertisement

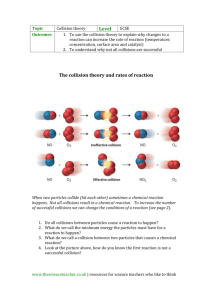
Rate of reaction 1 Measuring the rate of reaction It is the speed for a reactant to be used up or product to be formed. Rate of reaction (a) *describe the effect of concentration, pressure, particle size and temperature on the rates of reactions and explain these effects in terms of collisions between reacting particles (b) Define the term catalyst and describe the effect of catalysts (including enzymes) on the rates of reactions (c) *explain how pathways with lower activation energies account for the increase in rates of reactions (d) State that transition elements and their compounds act as catalysts (see 8.3) in a range of industrial processes and that enzymes are biological catalysts (e) Suggest a suitable method for investigating the effect of a given variable on the rate of a reaction (f)*interpret data obtained from experiments concerned with Ways to find the rate of reaction 1. Measuring the time for reaction to complete Speed of reaction is inversely proportional to time taken; the shorter the time needed for reaction to complete, the faster the speed of reaction is. rate of reaction Collision theory: (30 sec) for a reaction to occur particles should Collide with each other Collide with enough energy to overcome the activation energy for the reaction Collide in correct orientation. Grade 9 Chemistry 2016 © Gn.AEC (15 sec) 2 Rate of reaction 2. Measuring the amount of product produced in a period of time - Gradient largest at start indicating speed at its greatest. or measuring the amount of reactant remains in a period of time. - Gradient decreases with time – speed decreases with time. Can be measured by plotting change in volume of gas evolved, - Gradient becomes zero, speed is zero. The reaction has finished. mass of reaction mixture as reaction proceeds and change of pressure of gas formed. - Measuring the Change in mass in the reaction mixture - Measuring the amount of gas evolved. Consider reaction of limestone with acid to produce carbon dioxide. A syringe is used to help in measurement of gas produced in volume every time interval. A graph of volume of gas against time is plotted. Marble is reacted with acid in a flask with cotton wool stucked at top to prevent splashing during reaction but it allows gas to be free. The reading on balance is plotted on a graph on every time interval. Factors affecting the rate of reaction Grade 9 Chemistry 2016 © Gn.AEC Rate of reaction 3 Concentration: when concentration increases, the number of particles absorb the energy and having enough activation energy, particles per unit volume increases. Therefore frequency of they move faster and collide more effectively per second. collisions increases. Hence rate of reaction increases. Therefore, speed of reaction is increased. In the increase of concentration means there are more solute Usually, speed of reaction doubles for every 10oC rise in particles per unit volume of the solution which favours for more temperature. effective collision resulting in an increase in speed of reaction. Pressure: when pressure increases, the particles are forced Surface area: when surface area increases the number of closer together. Therefore frequency of collisions increases. Hence particles that can take part in the reaction increases. Therefore rate of reaction increases. frequency of collisions increases & rate of reaction increases. Only gaseous reactions are affected as gas is compressible. At When large marble is reacted with acid and compared to reaction of higher pressure, molecules are forced to move closely together, fine marble solids being reacted with acid and the graph of volume hence increasing the particles per unit volume of gas and effectively of gas against time is plotted, it’s found that the reaction involving increases the collision between reacting molecules so the speed of finer marble chips produces gas faster than the one with larger reaction increases. marble chunk as the graph of finer chips is steeper. The volume of High pressure is used in industrial processes (e.g. Haber Process gas at the end is the same for both reactions. Therefore, reactions Plant) so that the reaction goes faster. of solids with liquid/gas is faster when the solids are of smaller pieces. Temperature: when the temperature increase particles gain more kinetic energy and move faster. Frequency of collisions increases. Rate of reaction increases. Speed of reaction increases when temperature increases. Particles Catalyst: a catalyst is a substance that increases the rate of a don’t always react upon collision but just bounce as they don’t have chemical reaction, but is chemically unchanged at the end of the enough activation energy to react. With increase in temperature, Grade 9 Chemistry 2016 © Gn.AEC 4 Rate of reaction reaction. A catalyst provides an alternative route for the reaction with lower activation energy. It can be reused and only small amount of catalyst is needed to affect a reaction. - transition metals (e.g. Titanium, Nickel, Iron, Copper) are good catalysts - most catalyst catalyse one kind of reaction (except titanium) Catalysts lower the need of energy to break bonds so the activation energy needed is lower. Therefore, bond breaking in reactants occurs more often when particles collide. Factors Affecting Speed of Catalysed Reactions: Speed of catalyzed reaction can be increase by; Increasing the temperature, pressure and concentration Grade 9 Chemistry 2016 © Gn.AEC