Phase Changes & Heat Transfer Presentation
advertisement

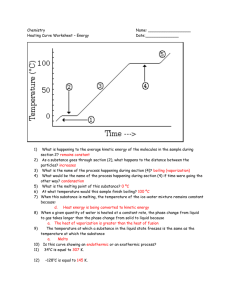
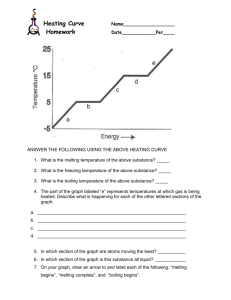
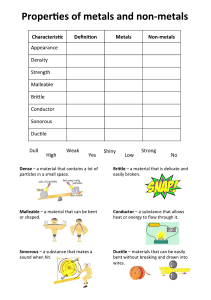
Phase Changes & Heat Transfer Energy Day 3 z z Warm-up! Individually, write down the answers to these two questions. 1. What does a “Phase Change” mean? 2. Describe what happens to particles when butter melts on popcorn. z Phase Changes z Phase Diagram z Heating Curve 1. Where is the melting point and boiling point on this diagram? 2. Why does the temperature stop increasing twice on the diagram? 3. Would this diagram look the same if we did the experiment on the top of a mountain where the atmospheric pressure is less? z Heating Curve of Water Heat of Fusion: The amount of energy needed at the melting point for the solid substance to become a liquid. Heat of Vaporization: The amount of energy needed at the boiling point for the liquid substance to become a gas. At the boiling point and melting point, the energy is absorbed as Potential Energy and NOT Kinetic Energy. Therefore the temperature does NOT change during the melting or boiliing process. Practice! z 1. What is the approximate temperature of the melting point and boiling point? 2. What is the approximate Energy in kJ of the Heat of Fusion and Heat of Vap.? z Instructions: (1) Read either the “Systems and Surroudings” Reading or the “Conduction, Convection, Radiation” Reading. (2) Focus on your assigned Vocabulary Word. (3) Agree with your partner or group on a summary of the vocabulary word to write on the Cue Card. (4) Wait for instructions on how we will share with the class.