Chemical Reagent Safety Data Sheet
advertisement
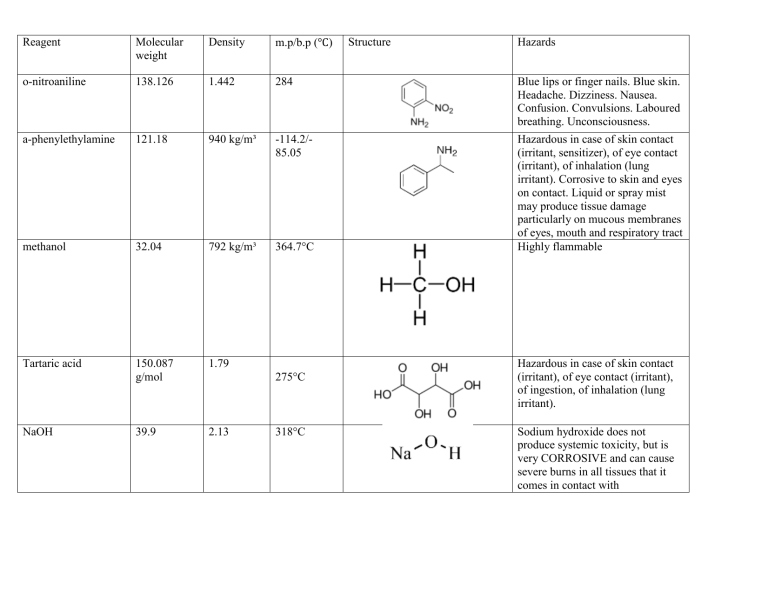
Reagent Molecular weight Density m.p/b.p (°C) o-nitroaniline 138.126 1.442 284 Blue lips or finger nails. Blue skin. Headache. Dizziness. Nausea. Confusion. Convulsions. Laboured breathing. Unconsciousness. a-phenylethylamine 121.18 940 kg/m³ -114.2/85.05 methanol 32.04 792 kg/m³ 364.7°C Hazardous in case of skin contact (irritant, sensitizer), of eye contact (irritant), of inhalation (lung irritant). Corrosive to skin and eyes on contact. Liquid or spray mist may produce tissue damage particularly on mucous membranes of eyes, mouth and respiratory tract Highly flammable Tartaric acid 150.087 g/mol 1.79 39.9 2.13 NaOH 275°C 318°C Structure Hazards Hazardous in case of skin contact (irritant), of eye contact (irritant), of ingestion, of inhalation (lung irritant). Sodium hydroxide does not produce systemic toxicity, but is very CORROSIVE and can cause severe burns in all tissues that it comes in contact with t-BuCl 92.6 840 kg/m3 51°C Highly flammable liquid and vapour. Cyclohexanol 100.158 962 kg/m3 161.8°C Contact can irritate and burn the skin and eyes. * Breathing Cyclohexanol can irritate the nose and throat. * High exposure can cause headache, nausea, vomiting, dizziness and passing out. 1-bromobutane 132.02 1.27 101.4°C Ingestion: May cause irritation of the digestive tract. May cause nausea and vomiting. May be harmful if swallowed. Inhalation: Causes respiratory tract irritation. H2SO4 98.079 1.84 10°C If sulfuric acid makes direct contact with the eyes, it can cause permanent blindness. If ingested, this chemical may cause internal burns, irreversible organ damage, and possibly death. Exposure to sulfuric acid aerosols at high concentrations leads to severe eye and respiratory tract irritation and tissue damage.