H2O
advertisement

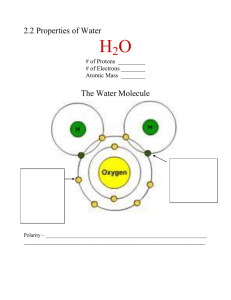
BIOL 110 Chapter 3 Water and Life ilcpblog.blogspot.com, funny-pictures.picphotos.net dataprocessingcenter.blogspot.com, www.slideshare.net Overview: Water •Water is the biological medium on Earth •All living organisms require water more than any other substance. •Most cells are surrounded by water, & cells themselves are about 70– 95% water www.earthtimes.org, kristinasuzannexo.blogspot.com Overview: Water • The abundance of water is the main reason the Earth is habitable • It is the only common substance to naturally exist in three physical states – liquid, solid (ice), and a gas (water vapor) biodiversity1.wordpress.com, www.wonderwhizkids.com Covalent Bonding H2O – • In a nonpolar covalent bond, the atoms share the electron equally O + H H + • In a polar covalent bond, 1 atom is more electronegative, & the atoms don’t share the electron equally • Unequal sharing of electrons causes a partial positive (+) or partial negative (-) charge for each atom or molecule (Slide # 20 from the previous lecture) Water Molecules – H-bonds • The water molecule is a polar molecule: the opposite ends have opposite charges • Polarity allows water molecules to form hydrogen bonds with each other Hydrogen bond + + Polar covalent bonds + + Water Properties Four of water’s properties that facilitate and allow an environment for life on earth are: 1. 2. 3. 4. Cohesive behavior Ability to moderate temperature Expansion upon freezing Versatility as a solvent http://clashot.com/editorial-photos/mobile/4807462.html Water: Cohesive Behavior • Collectively, hydrogen bonds hold water molecules together cohesion Adhesion Two types of water-conducting cells • Cohesion helps the transport of water against gravity in plants • Adhesion - attraction between different substances, Ex: between water & plant cell walls Cohesion Direction of water movement 300 m Water: Cohesive Behavior • • • Surface tension is a measure of how hard it is to break the surface of a liquid Surface tension is related to cohesion – at the water-air interface the molecules are orderly arranged through hydrogen bonds to one another and to the molecules below the surface Water behaves as if covered by an invisible film discoveryexpress.weebly.com, ouitschem.weebly.com , advchemyellow4.blogspot.com, apbio-werle.wikispaces.com Water: Moderation of Temperature • Water absorbs heat from warmer air & releases stored heat to cooler air • Water can absorb or release a large amount of heat with only a slight change in its own temperature • Anything that moves has kinetic energy – for instance atoms and molecules • Ek= mv2/2 • The Ek depends on the mass of the object (m) and the speed of the movement (v2) • The Ek of the random movement of atoms and molecules is called thermal energy Water: Moderation of Temperature • Temperature is the average kinetic energy of body of matter • The total thermal energy is proportional to the amount (the mass) of the matter • 200 ml of boiling water would have temperature of 100oC but less total thermal energy than a swimming pool with temperature around 20oC • Thermal energy transferred from one body of matter to another is defined as heat • Cub of ice in hot drink cools it by absorbing some of the thermal energy from the liquid that results in melting of the ice Water: Moderation of Temperature • The Celsius scale is a measure of temperature using Celsius degrees (C) • A calorie (cal) is the amount of heat required to raise the temperature of 1 g of water by 1°C • The “calories” on food packages are actually kilocalories (kcal), where 1 kcal = 1,000 cal • The Joule (J) is another unit of energy where 1 J = 0.239 cal, or 1 cal = 4.184 J Water’s High Specific Heat • The specific heat of a substance = the amount of heat that must be absorbed or lost for 1 g of that substance to change its temperature by 1ºC • The specific heat of water is 1 cal/g/ºC; ethyl alcohol has 0.6 cal/g/ºC; the Fe has 0.1 cal/g/ºC • Water resists changing its temp because of its high specific heat • Water’s high specific heat can be traced to hydrogen bonding • Heat is absorbed to break hydrogen bonds • Heat is released when hydrogen bonds form • The high specific heat of water minimizes temperature fluctuations to within limits that permit life. During the winter – the weather is milder closer to the ocean, and harsher inland. Water: Evaporative Cooling • Evaporation is transformation of a substance from liquid to gas • Heat of vaporization is the heat a liquid must absorb for 1 g to be converted to gas • To evaporate 1 g of H2O at 25oC about 580 cal are needed, nearly twice the amount needed to evaporate 1 g of alcohol or ammonia www.sciencedaily.com Water: Evaporative Cooling • As a liquid evaporates, its remaining surface cools, a process called evaporative cooling • Evaporative cooling of water helps stabilize temperatures in organisms and bodies of water www.trainingcor.com, www.vincentmartinez.info Floating of Ice on Liquid Water • Ice floats in liquid water because hydrogen bonds in ice are more “ordered,” making ice 10% less dense than liquid H2O at 4oC • Water reaches its greatest density at 4°C • If ice sank, all bodies of water would eventually freeze solid, making life impossible on Earth • What is the affect of global warming? Hydrogen bond Ice: Hydrogen bonds are stable Liquid water: Hydrogen bonds break and re-form Water: The Solvent of Life • • • • A solution is a liquid that is a homogeneous mixture of substances A solvent is the dissolving agent of a solution The solute is the substance that is dissolved An aqueous solution is one in which water = solvent www.youtube.com Water: The Solvent of Life • • Water is a versatile solvent due to its polarity, which allows it to form hydrogen bonds easily When an ionic compound is dissolved in water, each ion is surrounded by a sphere of water molecules called a hydration shell Na Cl www.biog1445.org, bio1151.nicerweb.com Na Cl Water: The Solvent of Life • Water can also dissolves compounds made of non-ionic polar molecules • Even large polar molecules (proteins) can dissolve in water if they have ionic & polar regions • Biological fluid as blood, cytoplasm, maple syrup are water-based solutions + + Water: The Solvent of Life • A hydrophilic substance is one that has an affinity for water • Some hydrophilic substances do not dissolve in water - cotton • A hydrophobic substance is one that does NOT have an affinity for water • Oil molecules - hydrophobic because they have relatively non-polar bonds • Fatty acids are main building blocks of the cell membranes www.brookstone.com, www.delish.com Water: The Solvent of Life Solute Concentration in Aqueous Solutions • Most biochemical reactions occur in water • Chemical reactions depend on collisions of molecules and therefore on the concentration of solutes in an aqueous solution chemwiki.ucdavis.edu Water: The Solvent of Life • When carrying out experiments, we use mass to calculate the number of molecules • Molecular mass = sum of all masses of all atoms in a molecule • Numbers of molecules are usually measured in moles, where 1 mole (mol) = 6.02 x 1023 molecules (Avogadro) • Avogadro’s number and the unit dalton were defined such that 6.02 x 1023 daltons = 1 g • Molarity (M) = number of moles of solute liter of solution Molarity and Avogadro's number Sucrose = C12H22O11 The molecular weight of sucrose will be: 12 times the atom mass of carbon(12) + 22 times the atom mass of H (1) + 11 times the atom mass of O (16) = 342 Daltons Because the way Daltons and Avogadros number are connected, to have 1 mol (6.02 x 1023 molecules) of sucrose we need to measure 342 g. Mol. Weight of NaCl = Na (23) + Cl (35.5) = 58.5 Daltons Molarity and Avogadro's number To prepare 1 M of Sucrose we need to take 342 g of that substance and dissolve it in 1 l of water. Workout: take 342 g sucrose, put in a flask and start adding water with constant stirring, until the entire amount of sucrose was dissolved. Then adjust the volume to 1 l 1 M of NaCl: measure 58.5 g solute, dissolve in 1 l of water. In both cases in the 1 l of solution we will have 6.02 x 1023 molecules in 1 l of sucrose or NaCl Acids & Bases and pH • A hydrogen atom in a hydrogen bond between 2 water molecules can shift from one to the other • • • • • The hydrogen atom leaves its electron behind & is transferred as a proton, or hydrogen ion (H+) The molecule with the extra proton is now a hydronium ion (H3O+), though it is often represented as H+ The molecule that lost the proton is now a hydroxide ion (OH–) Water is in a state of dynamic equilibrium in which water molecules dissociate at the same rate at which they are being reformed Only one out of every 554, 000, 000 is dissociated, the [] of each ion at 25oC is 10-7 M + 2 H 2O Hydronium ion (H3O+) Hydroxide ion (OH) Acids & Bases and pH • Though statistically rare, the dissociation of water molecules has a great effect on organisms • Changes in concentrations of H+ and OH– can drastically affect the chemistry of a cell • Concentrations of H+ and OH– are equal in pure water • Adding certain solutes, called acids and bases, modifies the concentrations of H+ and OH– • Biologists use the pH scale to describe whether a solution is acidic or basic • An acid is any substance that increases the H+ concentration of a solution • A base is any substance that reduces the H+ concentration of a solution Acids & Bases and pH • What and how the strong HCl does to the pH • HCl H+ + Cl- - results in increase of the H+ • What and how the strong NaOH does to the pH • NaOH Na+ + OH- - decrease of the H+ • Weak base: ammonia form ammonium ion • NH3 + H+ NH4+ - decrease of the H+ • Weak acid: carbonic acid form bicarbonate ion • H2CO3 HCO3- + H+ - increase of the H+ Acids & Bases and pH • In any aqueous solution at 25°C the product of H+ and OH– is constant & can be written as [H+][OH–] = 10–14 • In neutral solution (water) the [H+] = [OH–] = 10–7 • If acid is added and the [H+] goes up to 10–4 this implies that the [OH–] goes down to 10–10 • If base is added and the [OH–] goes up to 10–3 this implies that the [H+] goes down to 10–11 • Whenever we know the [H+] we can calculate the [OH–] Acids & Bases and pH Actually the concentrations are factor of 100 trillions or more This makes it unpractical for everyday use. In science we use the pH scale • The pH of a solution is defined by the negative logarithm of H+ concentration, written as • For a neutral aqueous solution pH = –log [H+] In neutral solution [H+] is 10–7 pH = -log 10-7 = –(–7) = 7 Each pH value (the [H+]) also implies the [OH-] value If the pH is 4, the [H+] is 10-4 and the [OH-] is 10-10 H+ H+ H+ OH + OH H + H H+ H+ H+ Acidic solution Increasingly Acidic [H+] > [OH] pH Scale 0 1 Battery acid 2 Gastric juice, lemon juice 3 Vinegar, wine, cola 4 Tomato juice Beer Black coffee 5 6 Neutral solution OH OH OH H+ OH OH OH OH + H Basic solution Neutral [H+] = [OH] 7 8 Increasingly Basic [H+] < [OH] OH OH H+ H+ OH OH OH + H H+ + H Rainwater Urine Saliva Pure water Human blood, tears Seawater Inside of small intestine 9 10 Milk of magnesia 11 Household ammonia 12 13 Household bleach Oven cleaner 14 pH Scale • Acidic solutions have pH values less than 7 • Basic solutions have pH values greater than 7 • Most biological fluids have pH values in the range of 6 to 8 Acids & Bases and pH 0 Acidic [H+] > [OH] Acids donate H+ in aqueous solutions. Neutral [H+] = [OH] 7 Basic [H+] < [OH] Bases donate OH or accept H+ in aqueous solutions 14 When the pH changes from 4 to 3 this is 10X increase in the concentration of the H+. Change from 3 to 6 is 1000X decrease of the H+. Acids, Bases, Buffers, and pH • The internal pH of most living cells must remain close to pH 7 • Buffers are substances that minimize changes in concentrations of H+ and OH– in a solution • Most buffers consist of a weak acid and its corresponding base, which combine reversibly with H+ • Ex. buffer in human blood and other biological solutions is carbonic acid (H2CO3), formed when CO2 reacts with water in blood plasma – Carbonic acid dissociates to yield a bicarbonate ion (HCO3-) and a hydrogen ion (H+) – The chemical equilibrium between carbonic acid and bicarbonate acts as a pH regulator – H2CO3 HCO3- + H+ – The equilibrium shifts left or right as other metabolic processes add or remove H+ from the solution Acidification of the Oceans • CO2 and the global warming • CO2 - H2CO3- • CaCO3 http://www.teachoceanscience.net/teaching_resources/education_modules/coral_reefs_and_climate_change/how_does_climate_change_affect_coral_reefs/