Lecture 3
advertisement
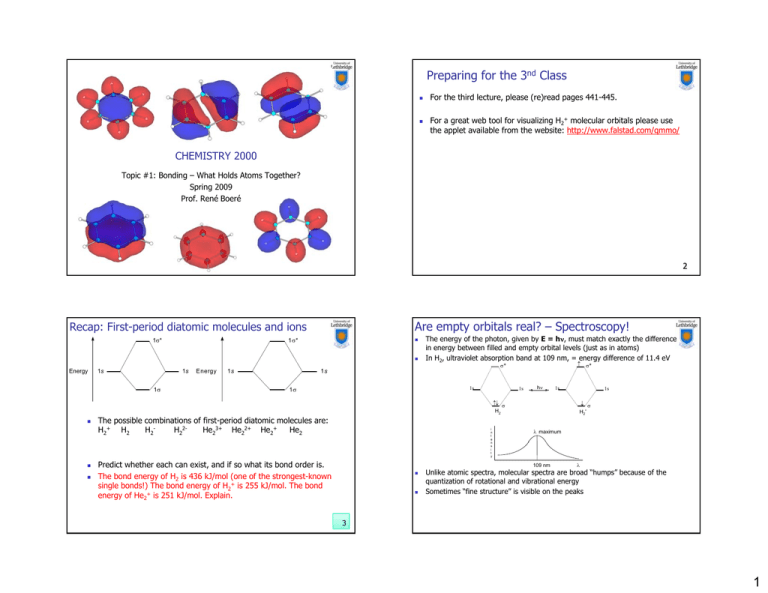
Preparing for the 3nd Class For the third lecture, please (re)read pages 441-445. For a great web tool for visualizing H2+ molecular orbitals please use the applet available from the website: http://www.falstad.com/qmmo/ http://www falstad com/qmmo/ CHEMISTRY 2000 Topic #1: Bonding – What Holds Atoms Together? Spring 2009 Prof. René Boeré 2 Are empty orbitals real? – Spectroscopy! Recap: First-period diatomic molecules and ions 1* 1* The energy of the photon, given by E = h, must match exactly the difference in energy between filled and empty orbital levels (just as in atoms) In H2, ultraviolet absorption band at 109 nm, = energy difference of 11.4 eV * Energy 1s 1s 1 Energy 1s 1s 1 1s h 1s H2 * 1s The possible combinations of first-period diatomic molecules are: H2H22He23+ He22+ He2+ He2 H2+ H2 i n t e n s i t y Predict whether each can exist, and iff so what its bond order is. The bond energy of H2 is 436 kJ/mol (one of the strongest-known single bonds!) The bond energy of H2+ is 255 kJ/mol. The bond energy of He2+ is 251 kJ/mol. Explain. H2* maximum 109 nm 1s Unlike atomic spectra, molecular spectra are broad “humps” because of the quantization of rotational and vibrational energy Sometimes “fine structure” is visible on the peaks 3 1 Molecular Orbitals of Homonuclear Diatomics Molecular Orbitals of Homonuclear Diatomics We can combine higher energy atomic orbitals in the same way. Compare the 2 and 2* orbitals in F2 to the 1 and 1* orbitals in H2: 1 or 1* p orbitals can also be combined to make molecular orbitals. The type of molecular orbital formed will depend on the orientation of the p orbitals. po orbitals b ta s tthat at o overlap e ap head-on ead o (usually (usua y defined de ed as tthe e pz orbitals) give molecular orbitals: 1s 1s 2 2s or 2* 2s What is the difference? 5 Molecular Orbitals of Homonuclear Diatomics Molecular Orbitals of Homonuclear Diatomics p orbitals that overlap side-on (usually defined as the px or py orbitals) give molecular orbitals: 6 …and here are the pretty computer-generated pictures of those orbitals: Combinations between s and p orbitals may also be possible. But CAUTION: I strongly recommend that you spend a good bit of time with the MO viewer applet, rotating the orbitals in three dimensions to really understand their shapes, and practice sketching these MO’s. 7 8 2 Why Pi? Molecular Orbitals of Homonuclear Diatomics Think about the bonding in N2. Drawing a Lewis structure tells us that the atoms are triple bonded and each has a lone pair. Each N atom starts with ____ electrons – ____ core electrons (which we ignore) and ____ valence electrons (____________). We can rotate each atom as much as we want, but we will never be b able bl to position i i them h such h that h we can make k three h bonds. At best, we could make two bonds, one with the 2s orbitals and one with a pair of 2p orbitals: Which p orbital combines with which other p orbital is symmetrydetermined. Since orthogonal orbitals on different atoms don’t combine, you won’t see combination of a px orbital on one atom and a py orbital on its neighbour, for example. s orbitals can combine with p orbitals when making MOs if the orbitals are Figure courtesy of Prof. Marc Roussel close enough in energy. The figure at the right shows 2s and 2p atomic orbital energies for the elements in period 2 2. We can see that there will be little mixing between 2s and 2p orbitals for the heavier elements in period 2. Using g orbitals,, we can find a wayy to make a triple p bond: 9 Choosing the right basis set 1. 2. Molecular Orbitals of Homonuclear Diatomics All atoms have infinite numbers of possible wavefunctions: which should we use to make bonds. There are two guiding principles: Those orbitals which are closest to each other in energy will undergo the greatest amount of interaction. Only orbitals within a narrow range of energy have the right properties to undergo significant interaction – the valence orbitals 0 H He Li Be B C N O F Ne Na 480 3s 2s 960 2s 1440 1s 2p O, F and Ne have large enough energy gaps between their 2s and 2p orbitals that they give the “normal” (valence) MO diagram: p bring together E N E R G Y 2u (2p)* 1g (2p)* 2p 2p 2g (2p) 2p 2s 2p 1u (2p) VALENCE ZONE 2p 1920 2p 2s 1u (2s)* 2p 2400 1s 2s 2p 2880 3360 3840 10 2s 2s 2s 1s 1g (2s) Energy level diagram 4320 S 12 3 What happens to the “core” orbitals in N2? Molecular Orbitals of Homonuclear Diatomics Each N atom has the electron configuration 1s22s22p3. The 1s level is “core”. O, F and Ne have large enough energy gaps between their 2s and 2p orbitals that they give the “normal” (valence) MO diagram: MO description of 2nd row homonuclear diatomic molecules bring together E N E R G Y 2u (2p)* 1g (2p)* 2p 2p 1u (2p) 2g (2p) 1u (2s)* 1 in H2 1 in H2 2s 1 in N2 1 in N2 1g (2s) 14 MO description of 2nd row homonuclear diatomic molecules 2u (2p)* Mixing in some “p character” lowers the energy of the 2* MO instead of E N E R G Y Mixing in some “s character” raises the energy of the 3 MO 2u Relative order may vary here 1g (2p)* Sigma and pi overlap leading to bonding and antibonding MO's Molecular Orbital Diagrams of Homonuclear Diatomics with the two observed energy sequences Because Li, Be, B, C and N have smaller energy gaps between their 2s and 2p orbitals, some mixing is observed when forming the and * orbitals, primarily 2* and 3: Energy level diagram 13 Molecular Orbitals of Homonuclear Diatomics 2s 2p 2p 1g (2p)* 1u (2p) Varying degree of s-p separation 2p 2g (2p) 2g Less s-p separation 1u (2s)* 2p 1u (2p) 1u instead of 2s 2s 2s 2s 1g (2s) If this effect is strong enough, the 3 orbital can end up higher in energy than the 1 orbital, giving the MO diagram on the next page. This is the case in Li2, Be2, B2, C2 and N2. 1g Energy levels in original diatomic construction 15 Energy levels in re-mixed diatomic 16 4 Applying the theory Applying the theory (2) Now determine possible bonding (or non-bonding) descriptions for all the second-row element diatomic molecules Li2 Be2 B2 C2 N2 Now determine possible bonding (or non-bonding) descriptions for all the second-row element diatomic molecules O2 F2 Ne2 17 Properties of known 2nd row diatomics 18 Application – Unexpected behaviour in O2 Property Li2 Be2 B2 C2 N2 O2 F2 Bond length (Å) 2.67 – 1.59 1.24 1.10 1.21 1.42 Bond energy (kJ mol–1) 110 – 272 602 941 493 138 1 0 1 2 3 2 1 Bond order Write the Lewis diagram for O2: Yet the MO diagram predicts the following valence electron configuration: What evidence can we use to decide the better description of the bonding? 20 5




