Numbers in Chemistry
advertisement

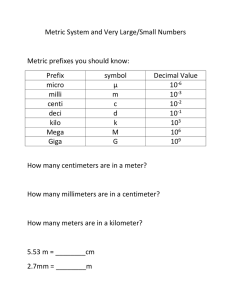
Numbers in Chemistry • Observations – ▫ Qualitative – quality of a characteristic Ex. Tall, short, cold, etc. ▫ Quantitative – Measureable quantity Ex. 25 cm, 50 m, 3.27 L SI Units • System International Quantity Unit Symbol Length Meter M Mass Kilogram Kg Time Second S Temperature Kelvin K Volume Liter L Amt of substance mole mol Metric Prefixes Prefix Symbol Meaning Mega M Million Kilo K Thousand Deci D Tenth Centi C Hundredth Milli m Thousandth Micro μ Millionth Nano n Billionth Pico p Trillionth Critical Thinking 1. How many milligrams in one kilogram? 2. How many meters are in 21.5 km? 3. Is it possible to answer this question: How many mg are in one km? 4. What is the difference between a Mm and a mm? Which is larger? Uncertainty in Measurement • A measurement always has some degree of uncertainty. Scientific Notation • Used to make very large or very small numbers easy to handle. • The Mole! • 6.02 x 1023 = 602,000,000,000,000,000,000,000 When using Scientific Notation, there are two kinds of exponents: positive and negative Positive Exponent: 2.35 x 108 Negative Exponent: 3.97 x 10-7 When changing scientific notation to standard notation, the exponent tells you if you should move the decimal: With a positive exponent, move the decimal to the right: 4.08 x 103 = 4 0 8 Don’t forget to fill in your zeroes! When changing scientific notation to standard notation, the exponent tells you if you should move the decimal: With a negative exponent, move the decimal to the left: 4.08 x 10-3 = 408 Don’t forget to fill in your zeroes! An easy way to remember this is: • If an exponent is positive, the number gets larger, so move the decimal to the right. • If an exponent is negative, the number gets smaller, so move the decimal to the left. The exponent also tells how many spaces to move the decimal: 4.08 x 103 = 4 0 8 In this problem, the exponent is +3, so the decimal moves 3 spaces to the right. The exponent also tells how many spaces to move the decimal: 4.08 x 10-3 = 408 In this problem, the exponent is -3, so the decimal moves 3 spaces to the left. Try changing these numbers from Scientific 1) 8.41 x 10 Notation to-7Standard Form 2) 3.215 x 108 Try changing these numbers from Standard Form to Scientific Notation 1) 25, 310, 000, 000, 000, 000 2) 0.000000003018 When changing from Standard Notation to Scientific Notation: 1) First, move the decimal after the first whole number: 3258 2) Second, add your multiplication sign and your base (10). 3 . 2 5 8 x 10 3) Count how many spaces the decimal moved and this is the exponent. 3 . 2 5 8 x 10 3 3 2 1 Mathematic Operations and Scientific Notation. • Multiply – Add Exponents • Divide – Subtract Exponents • (4.6 x 1034) (7.9 x10-21) • 8.4x 10-4/4.1 x 1017 Calculator Operations • • • • • • Calculators make it easy. Problem: (1.24 x 1012) (3.31 x 1020) Type in 1.24 EE 12 x 3.31 EE 20 Enter Try it! Problem : 5.4 x 1032/ 7.3 x1014 Type in 5.4 EE 32 / 7.3 EE 14 Enter Adding or Subtracting • Make sure the exponents are the same OR use the EE button! • 4.25 x 1013 + 2.10 x 1014 • 6.4 x10 -18 – 3 x 10-19 • 3.1 x 10-34 + 2.2 x 10-33 Uncertainty in Measurement • Different people estimate differently. • Record all certain numbers and one estimated number. Significant Figures • Numbers recorded in a measurement. ▫ All the certain numbers plus first estimated number Significant Figures Rules for Counting Significant Figures 1. Nonzero integers always count as significant figures. 1457 4 significant figures Significant Figures Rules for Counting Significant Figures 2. Zeros a. Leading zeros - never count 0.0025 2 significant figures b. Captive zeros - always count 1.008 4 significant figures c. Trailing zeros - count only if the number is written with a decimal point 100 1 significant figure 100. 3 significant figures 120.0 4 significant figures Significant Figures Rules for Counting Significant Figures 3. Exact numbers - unlimited significant figures • Not obtained by measurement • Determined by counting 3 apples • Determined by definition 1 in. = 2.54 cm Significant Figures Significant Figures Rules for Multiplication and Division • The number of significant figures in the result is the same as in the measurement with the smallest number of significant figures. Significant Figures Rules for Addition and Subtraction • The number of significant figures in the result is the same as in the measurement with the smallest number of decimal places.