Average Atomic Mass and Isotopes
advertisement

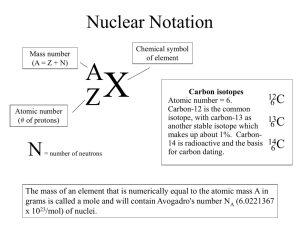
Name__________________________________________Date_______________Period_______ Average Atomic Mass and Isotopes • Go to the University of Colorado – Boulder PhET website at http://phet.colorado.edu • Click “Play with Sims” • Seek and click on “Chemistry” on the left-hand panel • Find and click on “Isotopes and Atomic Mass” • Click Run Now! • Make sure Make Isotopes is selected • Click on the plus signs next to Symbol and Abundance in Nature is selected • Click on the radio button on the scale next to Atomic Mass (amu) Hydrogen 1. What is the atomic mass of hydrogen-1? ____________What is its abundance?________ 2. Add a neutron, what is the mass?____________ What is the abundance?_____________ 3. Add one more neutron. What happens to the isotope? ____________________________ ___________________________________________________________________________ What is the mass?_______________________ The abundance?_______________________ 4. Calculate the average atomic mass of hydrogen using the atomic mass and abundance of each isotope. Note: the abundance of hydrogen-3 is so small that we do not need to include it in our equation. SHOW YOUR WORK!! Neon 1. Click on Neon on the periodic table 2. What is the abundance of Neon-20?_________________ What is the mass?___________ 3. What happens to the atom if you remove a neutron? ______________________________ ___________________________________________________________________________ 4. Create Neon-21. How many neutrons are in this isotope?_____ What is the mass?______ ____________What is the abundance?________________ 5. Add one more neutron. What is the mass?_________ What is the abundance?_________ 6. Calculate the average atomic mass of Neon. SHOW YOUR WORK!! Leave four decimal places. Click on the tab: Mix Isotopes Hydrogen 1. Add one Hydrogen-1, what is its mass? _______________Remove it and add one Hydrogen-2. What is its mass?_____________________ 2. What was the mass you calculated from question four under Hydrogen?______________ Which isotope is the “normal” isotope?________________________________________ 3. Reset All. In order to discover the relationship between percent composition and average atomic mass, it is helpful to be more systematic when choosing the number of atoms in the simulation. Complete the following table by adding purple and green atoms to the black box. In order to add larger amounts, Click “More” and use the slider bar or numerically enter data. # of atoms Hydrogen-1 Purple 1 # of atoms Hydrogen-2 Green 1 5 5 5 10 10 20 1 10 10 1 20 1 50 1 % Hydrogen-1 % Hydrogen-2 Average Atomic Mass Purple Green (amu) 4. The average atomic mass for hydrogen is listed as 1.007 amu on the periodic table. Predict the combination of purple and green atoms required to achieve this mass._____________________________ (The most you can have of each atom is 99.) How close can you get to the mass of Hydrogen?____________________________________________________________________________ 5. Check your prediction by clicking on “Nature’s mix of isotopes”. Were you right?__________________ 6. Look at your table, what conclusions can you draw about the relationship between the relative abundances and the average atomic mass? _____________________________________________________________ ________________________________________________________________________________________ Magnesium 1. Weigh each isotope of Magnesium. Magnesium-24_________ Magnesium-25_________ Magnesium26_____________ The atomic mass of Magnesium is 24.31. Which isotope is the “normal” isotope? ______________________________________________ 2. Use the percent abundances and the masses of each isotope to calculate the average atomic mass. Is it the same as the mass from the periodic table? ____________________________SHOW YOUR WORK! Show work here: Conclusion Questions 1. Carbon has an average atomic mass of 12.011 amu (as given on the periodic table). Which isotope of carbon do you think is most abundant: carbon-12 or carbon-13? ______________ Explain your answer. ______________________________________________________________________________________ ______________________________________________________________________________________ ______________________________________________________________________________________ 2. Define average atomic mass in your own words. _______________________________________________ ______________________________________________________________________________________ 3. Carbon has three isotopes. Carbon-12 has a natural abundance of 98.93% and a weight of 12.000 amu. Carbon-13 has an abundance of 1.07% and a weight of 13.00335. Carbon-14 has an abundance that is too small to matter. Calculate the average atomic mass.